Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tryptophan metabolism plays an essential role in human health. In mammals, about 95% of dietary tryptophan is metabolized through the kynurenine pathway, which is associated with the development of several pathologies, including neurodegeneration. Some of the kynurenine pathway metabolites are agonists of the aryl hydrocarbon receptor involved in metabolic functions, inflammation, and carcinogenesis.
- tryptophan metabolites
- kynurenine pathway
- kynurenine
1. Introduction
Tryptophan (TRP) is an exogenous amino acid that cannot be synthesized in the human body. It must be delivered through nutritional sources and, in the organism, it is found bound to albumin or free form [1]. In the human organism, TRP is involved in the biosynthesis of protein and regulation of metabolic networks as it is a precursor for important biologically active compounds like coenzymes nicotinamide adenine dinucleotide (NAD+), and NAD phosphate (NADP+), serotonin, tryptamine, melatonin, niacin, kynurenine (KYN), and indole and indolic acid derivatives [2][3]. Its concentration in the body is lower than that of other amino acids and it might play a rate-limiting role in protein synthesis [4]. The gut microbiota utilizes ~4–6% of TRP for the production of indole, indican, tryptamine, skatole, and indole acid derivatives [5]. In the mammal’s brain, TRP is metabolized to serotonin (5-hydroxytryptamine)—a neurotransmitter that modulates neural activity and a wide range of neuropsychological processes like mood, perception, reward, anger, aggression, appetite, memory, sexuality, and attention [6]. In the pineal gland, serotonin serves as a precursor for the synthesis of melatonin, which is involved in the regulation of circadian rhythm, and the reproductive and immune systems [7]. Approximately, 3% of dietary TRP is utilized for serotonin synthesis throughout the body (1% is utilized in the brain) [8]. Alternatively, TRP might be converted to a trace amount of tryptamine—an important neuromodulator of serotonin [3]. About 95% of intake TRP is metabolized via the kynurenine pathway (KP), producing KYN and other derivatives [9]. KYN secretion by different cells plays an important role in immune privilege during infections, inflammations, pregnancy, and cancer. The contributions of the different metabolic pathways of TRP utilization may differ under physiological and pathological statuses.
Both excessive intake and deficiency of TRP can unbalance its homeostasis and affect human health [4]. The impact of TRP supplementation on human health has so far been studied mainly in terms of serotonin pathway activation. The benefits of TRP loading on human cognition, mood, and sleep as a result of serotonergic stimulation have been widely reported [10]. The KP is the main metabolic route of this amino acid. Moreover, food might contain KP metabolites itself, which has been confirmed by many studies [11][12][13][14][15][16][17][18][19]. Some of them are essential for the proper functioning of the body and have a beneficial effect, e.g., kynurenic acid (KYNA) or nicotinamideadenine dinucleotide. On the other hand, KP delivers some metabolites which could exert cytotoxic (e.g., KYN, 3-hydroxyanthranilic acid (3HAA), and 3-hydroxykynurenine (3HKyn)) and neurotoxic (e.g., quinolinic acid (QA)) impacts. Notably, elevated levels of some KP metabolites are associated with various diseases, e.g., increased KYN and KYNA are connected with inflammatory bowel disease [20] and ulcerative colitis [21], respectively. Providing compounds from KP with food can positively or negatively affect human health.
2. Kynurenine Pathway—An Overview
In mammals, KP (Figure 1) is initiated through the activity of the enzymes: indoleamine-2,3-dioxygenases (IDO1 and IDO2) or tryptophan-2,3-dioxygenase (TDO). TDO acts mainly in the liver, while IDO has a wide tissue and cellular distribution. As a result of this reaction, N-formylkynurenine (NFK) is formed which is instantaneously converted by NFK formamidase to L-KYN—the first stable KP metabolite [22]. Degradation of L-KYN proceeds in three ways: (1) enzymes family of kynurenine aminotransferase (KAT) leads to the production of KYNA; (2) L-KYN can be hydrolyzed by kynureninase to anthranilic acid (AA); and (3) kynurenine mono-oxygenase (KMO) can lead to hydroxylation of L-KYN resulting in 3HKyn formation. The activity of some KP enzymes (IDO and KMO) is also upregulated by immune stimulation [23]. Further transformation of 3HKyn is accomplished by kynureninase and causes 3HAA formation [24]. The next product, 2-amino-3-carboxymuconic 6-semialdehyde (ACMS), is formed as a result of 3-hydroxyanthranilate-3,4-dioxygenase action. This unstable metabolite can be converted in two different ways: (1) preferably via QA, which, subsequently, undergoes the transformation and leads to the production of the essential redox cofactor nicotinamideadenine dinucleotide (NAD+) and (2) less efficiently 2-amino-3-muconic acid-6-semialdehyde (AMS), which, subsequently, generates picolinic acid (PIC) resulting from non-enzymatic cyclization or it is metabolized to acetyl CoA [22][25]. Alternatively, the transamination of 3HKyn by KAT leads to xanthurenic acid (XA) formation, whereas the dimerization of 3HAA produces cinnabarinic acid (CA) [23].
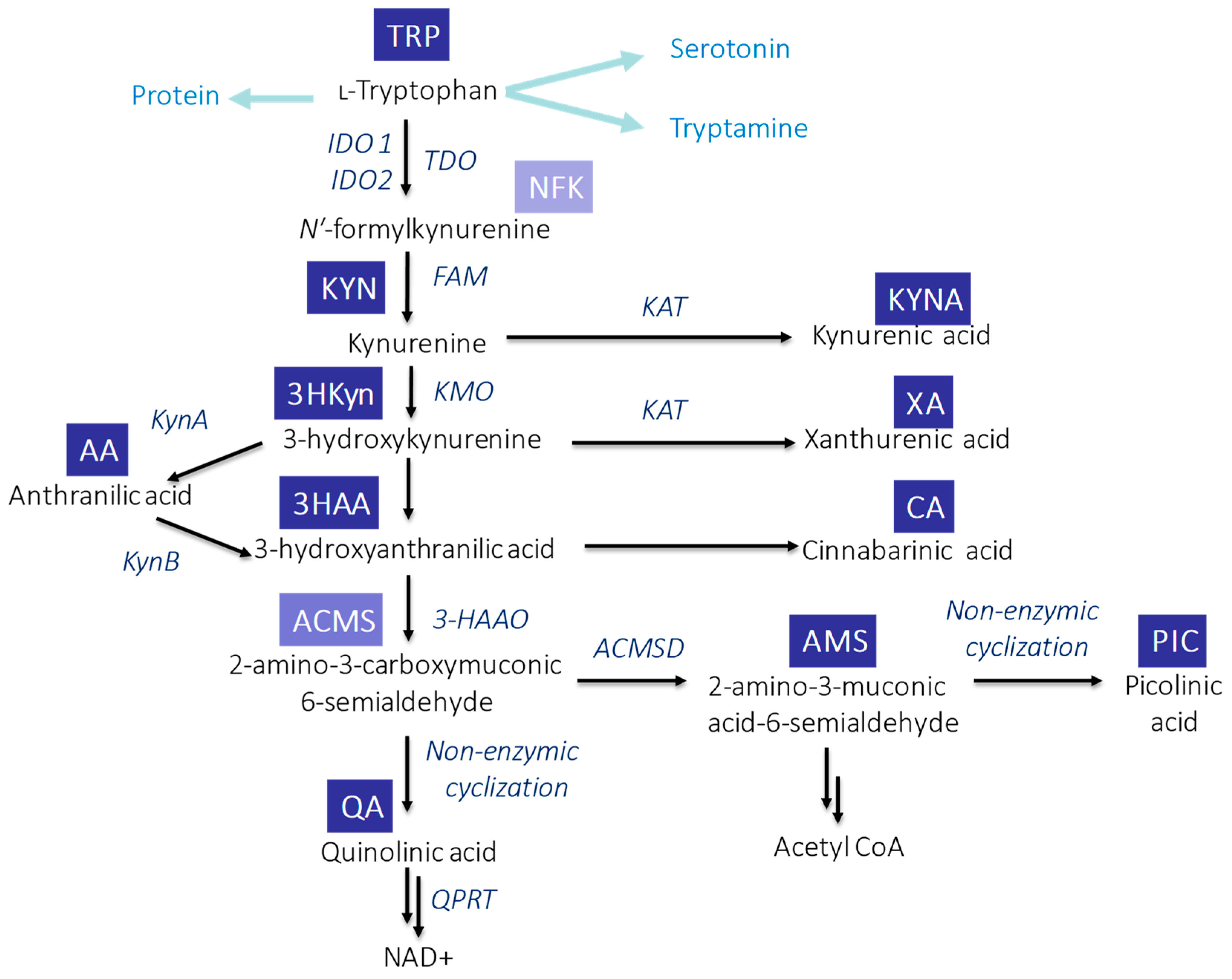
Figure 1. Schematic overview of TRP metabolism via KP in mammals. Abbreviations: ACMSD—2-amino-3-carboxymuconic acid semialdehyde decarboxylase; FAM—N′-formylkynurenineformamidase; 3-HAAO—3-hydroxyanthranilic acid 3,4-dioxygenase; IDO1—indoleamine-2,3-dioxygenase 1; IDO1—indoleamine-2,3-dioxygenase 2; KAT—kynurenine aminotransferase; KMO—kynurenine mono-oxygenase; KynA—kynureninase A; KynB—kynureninase B; QPR—quinolinate phosphoribosyl transferase; and TDO—tryptophan-2,3-dioxygenase.
3. Circulation of KP Metabolites
In the human body, approximately <1% of ingested TRP is utilized for protein synthesis. TRP is transported by large neutral amino acid transporters mainly into the gut, where it is metabolized by microbiota [5]. The rest enters portal circulation and undergoes liver metabolism [26]. Notably, about 75–95% of circulating TRP is bound to albumin, but only free TRP can cross the blood–brain barrier (BBB). The unbounded TRP can be further metabolized along four degradation pathways [3][26].
Gut microbiota modulates intestinal TRP metabolism. Commensal microbes can transform TRP into tryptamine (by tryptophan decarboxylases), indole and its derivatives (by tryptophanase), and serotonin (by tryptophan synthetase). However, the initiation of TRP degradation via KP occurs by the activation of Toll-like receptors (TLRs) by microbial components. Notably, butyrate—produced by gut microbes—suppresses KYN production by downregulation of intestinal IDO expression via various mechanisms [27].
More than 60% of KYN is directly transported from peripheral circulation to the brain, where it is transformed into other neuroactive compounds [28]. 3HKyn and AA also have the ability to cross BBB, while KYNA, 3HAA, and QA cross it very poorly [28][29][30]. Furthermore, KYN can cross the placenta and fetal blood–brain barrier [31].
In peripheral tissues (liver and kidney), phagocytes (monocytes and macrophages), and microglia cells, KMO predominantly break down KYN to 3HKyn, which is further cleavaged to 3HAA and QA, leading to NAD+ formation [23]. Alternatively, KYN can be hydrolyzed to AA—a precursor for QA production. In astrocytes, however, KAT is catalyzed to KYN and converted to KYNA. Furthermore, glial cells and neurons produce PIC [23].
After oral ingestion, KYN is absorbed into the intestine [28]. Also, KYNA can be easily absorbed from the lumen of the digestive system into the bloodstream and transported to the liver and the kidneys [32][33]. In blood, its concentration achieves the highest level 15–30 min after ingestion, and back to the basal level after 2 h [33]. After ingestion, a successive increase in KYNA level in bile, pancreatic juice, and intestinal lumen is observed [34]. Under normal conditions, the majority of KP metabolites are excreted in the urine [34][35][36]. The presence of some KP metabolites in faeces and sweat was also demonstrated [34][36].
4. Biological Functions of KP Metabolites
The role of KP is connected with many diseases and conditions. The metabolism of TRP is related to neuropsychiatric and cardiovascular diseases, inflammation, and cancer, and KP metabolites have various biological functions (Table 1).
Table 1. Comparison of positive and negative health effects of the KP metabolites.
| Biological Effect | KP Metabolite | Ref. |
|---|---|---|
| Anticonvulsant properties | KYNA, PIC | [23] |
| Anti-inflammatory properties | 3HAA, 3HKyn | [23][37] |
| Antimicrobial activity | KYN, KYNA, CA, PIC | [23][38] |
| Antioxidant properties | KYNA, XA, AA, 3HKyn | [23][37][39] |
| Antiviral properties | PIC | [23] |
| Immunomodulation | KYN, CA | [23][38] |
| Lipid peroxidation | QA | [40] |
| Neurotoxicity | 3HKyn, QA | [37][40] |
| Neuroprotective properties | KYNA | [23] |
| Oxidative stress regulation | KYN, 3HKyn, 3HAA | [23] |
| Proconvulsant properties | QA | [23] |
| Pro-oxidant properties | 3HKyn, QA | [23][37] |
| Reduction of lipid peroxidation | KYNA | [23] |
| Transcription factor | KYN | [23] |
| Vasodilator in endothelial cells | KYN | [23] |
KYN shows immudulatory properties, e.g., it inhibits T-cell proliferation, reduces the activity of natural killer cells and dendritic cells, and promotes the differentiation of regulatory T-cells [41][42][43]. This metabolite shows antimicrobial activity and was identified as a specific agonist of the human aryl hydrocarbon receptor (AhR) [38]. Upregulation of KYN was noted in the case of such pathologies as infections, autoimmunological diseases, and cancer [44]. An increasing ratio of KYN/TRP is associated with cardiovascular diseases such as coronary heart disease [45][46] and it can be considered a biomarker for irritable bowel syndrome. Of note, it was demonstrated in rats, that this KP metabolite mediates vasorelaxation and lower blood pressure in a dose-dependent manner [47]. Furthermore, KYN production in the human body also varies when neurological and psychiatric disorders occur [48].
From a therapeutic standpoint, KYNA is one of the most interesting metabolites [23]. It is mainly produced in glial cells. It is also a competitive antagonist of glutamate receptors—NMDA receptors, kainate receptors, and AMPA receptors. The additional targets of KYNA are the α7 nicotinic acetylcholine receptor (α7nAChR), the former orphan G protein-coupled receptor (GPR35), and AhR [29]. KYNA shows neuroprotective, antioxidant-free radical scavenging properties, reduces oxidative stress, and decreases protein and lipid damage [23]. There are strong indications that this TRP metabolite exerts anti-inflammatory or proinflammatory effects, depending on whether inflammatory or homeostatic conditions are considered [49]. It was demonstrated on human natural killer T-cells that activation of GPR35 by KYNA (300 µM) significantly reduces the release of IL-4 [50]. This KP metabolite of ~500 µM dose suppresses the production of IL-23 and IL-17 by dendritic cells [51]. KYNA inhibits TNF-α release from human mononuclear leukocytes [52]. It was also demonstrated that cancer cells in the intestine produce KYNA more effectively in comparison with normal colon epithelial cells, without overexpression of KATs in these cell lines [53]. The antiproliferative action of KYNA (>10 µM) against in vitro cultured colon cancer cells (HT-29, LS-180, and Caco-2) has also been reported [53]. On the other hand, KYNA could influence intestinal inflammation and hypermotility [54]. An increased ratio of KYNA/TRP is associated with endoscopic inflammation and is predictive of disease outcomes in ulcerative colitis patients [21]. A higher level of KYNA was also noted in patients with schizophrenia relative to healthy controls (when KYNA content was analyzed in cerebrospinal fluid, central nervous system, and brain tissue samples) [55]. On the other hand, a decrease in KYNA occurs in patients with Parkinson’s and Huntington’s diseases, and multiple sclerosis [29].
3Hkyn and 3HAA are both good electron donors and their oxidation results in the formation of a highly reactive quinoneimine [37]. Even the micromolar concentration of these compounds contributes to the generation of the reactive oxygen species (ROS)—hydroxyl radicals and hydrogen peroxides—and induces oxidative stress [56]. This pro-oxidant behavior would explain some toxical actions of 3HAA and 3HKyn [42][57][58]. In particular, they are T-cell-suppressive and have an additive effect in the presence of KYN [42]. Furthermore, a large body of literature has demonstrated in vitro neurotoxicity of 3Hkyn by ROS overproduction, direct protein interaction, and mitochondrial dysfunction [37]. On the contrary, many reports reveal the antioxidant function of both 3HAA and 3HKyn [37][57]. For example, these two metabolites of 20 µM dose effectively protected B-phycoerythrin from peroxyl radical-mediated oxidative damage [59]. Increasing 3HKyn and 3HAA can be related to neurodegenerative disorders—Huntington’s, Parkinson’s, and Alzheimer’s diseases. Another KP metabolite—AA—could be considered a biological marker in schizophrenia [60] or gastric cancer patients [61]. Although AA is not efficient as a peroxyl radical scavenger, this KP metabolite acts as the chelating agent of Cu(II) ions and is a good protector against the hydroxyl radicals associated with oxidative stress through its secondary antioxidant activity [39].
QA is a well-known neurotoxin produced in the brain [40][62]. It can be a neuroprotective compound but only in low concentration. Increasing QA causes saturation of the catabolic system and this condition becomes toxic [63]. QA exerts neurotoxic effects by several mechanisms including activation of the N-methyl-d-aspartate (NMDA) receptor in pathophysiological concentrations [40]. Elevated levels of QA are noted to be in the case of patients with Alzheimer’s disease and inflammatory disorders. In Huntington’s disease, the increase in the brain QA is accompanied by an increase in cerebral 3Hkyn and a reduction in the brain KYNA [29]. Neurotoxicity of QA is associated with the pathogenesis of neurodegenerative processes connected with neuroinflammation [64]. Therefore, it is associated with such diseases as multiple sclerosis and Huntington’s disease. In vitro experiments demonstrated that QA does not induce the death of lymphocytes [42]. However, in TRP-free extracellular microenvironment, inhibition of T-cell proliferation could be observed [43].
KP by-products—XA and CA—are neuroactive compounds that modulate metabotropic glutamate (mGlu) receptors [23][65][66]. Studies performed on schizophrenia patients have shown a decrease in XA levels in serum compared to healthy controls [67]. This metabolite shows anticonvulsant and antioxidant properties [23]. The formation of XA is believed to be a major detoxification route for 3HKyn production [37]. Notably, CA is a ligand of AhR [65], and shows antibacterial properties [68].
3HAA, AA, and CA are also important players in the shikimic acid pathway [69], allowing the biosynthesis of aromatic amino acids in fungi, bacteria, and plants.
PIC is utilized for the production of local anesthetics or supplements [70], shows antiviral, antifungal, antitumoral properties, and regulates cell growth. In the human body, this compound plays the role of a chelating agent of such elements as zinc, copper, iron, molybdenum, and manganese [71]. It inhibits the proliferation of peripheral blood leukocytes [43].
This entry is adapted from the peer-reviewed paper 10.3390/ijms242216304
References
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 1–10.
- Chen, Y.; Chen, H.; Shi, G.; Yang, M.; Zheng, F.; Zheng, Z.; Zhang, S.; Zhong, S. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Quantitative Profiling of Tryptophan Metabolites in Human Plasma and Its Application to Clinical Study. J. Chromatogr. B 2019, 1128, 121745.
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60.
- La Scala, J.J.; Sands, J.M.; Palmese, G.R. L-Tryptophan: Biochemical, Nutritional and Pharmacological Aspects. AIChE Annu. Meet. Conf. Proc. 1996, 10, 21–47.
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13.
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366.
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906.
- Sandyk, R. L-Tryptophan in Neuropsychiatry Disorders: A Review. Int. J. Neurosci. 1992, 67, 127–144.
- Takikawa, O. Biochemical and Medical Aspects of the Indoleamine2,3-Dioxygenase-Initiated L-Tryptophan Metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19.
- Silber, B.Y.; Schmitt, J.A.J. Effects of Tryptophan Loading on Human Cognition, Mood, and Sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407.
- Turska, M.; Rutyna, R.; Paluszkiewicz, M.; Terlecka, P.; Dobrowolski, A.; Pelak, J.; Turski, M.P.; Muszyńska, B.; Dabrowski, W.; Kocki, T.; et al. Presence of Kynurenic Acid in Alcoholic Beverages—Is This Good News, or Bad News? Med. Hypotheses 2019, 122, 200–205.
- Turska, M.; Pelak, J.; Turski, M.P.; Kocki, T.; Dukowski, P.; Plech, T.; Turski, W. Fate and Distribution of Kynurenic Acid Administered as Beverage. Pharmacol. Rep. 2018, 70, 1089–1096.
- Milart, P.; Paluszkiewicz, P.; Dobrowolski, P.; Tomaszewska, E.; Smolinska, K.; Debinska, I.; Gawel, K.; Walczak, K.; Bednarski, J.; Turska, M.; et al. Kynurenic Acid as the Neglected Ingredient of Commercial Baby Formulas. Sci. Rep. 2019, 9, 6108–6115.
- Turski, M.P.; Chwil, S.; Turska, M.; Chwil, M.; Kocki, T.; Rajtar, G.; Parada-Turska, J. An Exceptionally High Content of Kynurenic Acid in Chestnut Honey and Flowers of Chestnut Tree. J. Food Compos. Anal. 2016, 48, 67–72.
- Soto, M.E.; Ares, A.M.; Bernal, J.; Nozal, M.J.; Bernal, J.L. Simultaneous Determination of Tryptophan, Kynurenine, Kynurenic and Xanthurenic Acids in Honey by Liquid Chromatography with Diode Array, Fluorescence and Tandem Mass Spectrometry Detection. J. Chromatogr. A 2011, 1218, 7592–7600.
- Bertazzo, A.; Ragazzi, E.; Visioli, F. Evolution of Tryptophan and Its Foremost Metabolites’ Concentrations in Milk and Fermented Dairy Products. PharmaNutrition 2016, 4, 62–67.
- Turski, M.P.; Kamiński, P.; Zgrajka, W.; Turska, M.; Turski, W.A. Potato- An Important Source of Nutritional Kynurenic Acid. Plant Foods Hum. Nutr. 2012, 67, 17–23.
- Yılmaz, C.; Gökmen, V. Determination of Tryptophan Derivatives in Kynurenine Pathway in Fermented Foods Using Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2018, 243, 420–427.
- Turski, M.P.; Turska, M.; Kocki, T.; Turski, W.A.; Paluszkiewicz, P. Kynurenic Acid Content in Selected Culinary Herbs and Spices. J. Chem. 2015, 2015, 617571.
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD+ Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497.
- Sofia, M.A.; Ciorba, M.A.; Meckel, K.; Lim, C.K.; Guillemin, G.J.; Weber, C.R.; Bissonnette, M.; Pekow, J.R. Tryptophan Metabolism through the Kynurenine Pathway Is Associated with Endoscopic Inflammation in Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1471–1480.
- Maddison, D.C.; Giorgini, F. The Kynurenine Pathway and Neurodegenerative Disease. Semin. Cell Dev. Biol. 2015, 40, 134–141.
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548.
- Badawy, A.A.B. Kynurenine Pathway and Human Systems. Exp. Gerontol. 2020, 129, 110770.
- Badawy, A.A.B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1–10.
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794.
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723.
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine Emerges from the Shadows—Current Knowledge on Its Fate and Function. Pharmacol. Ther. 2021, 225, 107845.
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477.
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood–Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2016.
- Goeden, N.; Notarangelo, F.M.; Pocivavsek, A.; Beggiato, S.; Bonnin, A.; Schwarcz, R. Prenatal Dynamics of Kynurenine Pathway Metabolism in Mice: Focus on Kynurenic Acid. Dev. Neurosci. 2017, 39, 519–528.
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the Digestive System—New Facts, New Challenges. Int. J. Tryptophan Res. 2013, 6, 47–55.
- Turski, M.P.; Turska, M.; Zgrajka, W.; Kuc, D.; Turski, W.A. Presence of Kynurenic Acid in Food and Honeybee Products. Amino Acids 2009, 36, 75–80.
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 12, 4182.
- Pawlak, D.; Tankiewicz, A.; Matys, T.; Buczko, W. Peripheral Distribution of Kynurenine Metabolites and Activity of Kynurenine Pathway Enzymes in Renal Failure. J. Physiol. Pharmacol. 2003, 54, 175–189.
- Saran, T.; Turska, M.; Kocki, T.; Zawadka, M.; Zieliński, G.; Turski, W.A.; Gawda, P. Effect of 4-Week Physical Exercises on Tryptophan, Kynurenine and Kynurenic Acid Content in Human Sweat. Sci. Rep. 2021, 11, 11092–11098.
- Colín-González, A.L.; Maldonado, P.D.; Santamaría, A. 3-Hydroxykynurenine: An Intriguing Molecule Exerting Dual Actions in the Central Nervous System. Neurotoxicology 2013, 34, 189–204.
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203.
- Francisco-Marquez, M.; Aguilar-Fernández, M.; Galano, A. Anthranilic Acid as a Secondary Antioxidant: Implications to the Inhibition of Radical OH Production and the Associated Oxidative Stress. Comput. Theor. Chem. 2016, 1077, 18–24.
- Guillemin, G.J. Quinolinic Acid, the Inescapable Neurotoxin. FEBS J. 2012, 279, 1325–1365.
- Sadok, I.; Staniszewska, M. Electrochemical Determination of Kynurenine Pathway Metabolites—Challenges and Perspectives. Sensors 2021, 21, 7152.
- Terness, P.; Bauer, T.M.; Röse, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of Allogeneic T Cell Proliferation by Indoleamine 2,3-Dioxygenase–Expressing Dendritic Cells. J. Exp. Med. 2002, 196, 447–457.
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-Derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468.
- Metcalfe, A.J.; Koliamitra, C.; Javelle, F.; Bloch, W.; Zimmer, P. Acute and Chronic Effects of Exercise on the Kynurenine Pathway in Humans—A Brief Review and Future Perspectives. Physiol. Behav. 2018, 194, 583–587.
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune Activation and Degradation of Tryptophan in Coronary Heart Disease. Eur. J. Clin. Investig. 2003, 33, 550–554.
- Qi, Q.; Hua, S.; Clish, C.B.; Scott, J.M.; Hanna, D.B.; Wang, T.; Haberlen, S.A.; Shah, S.J.; Glesby, M.J.; Lazar, J.M.; et al. Plasma Tryptophan-Kynurenine Metabolites Are Altered in Human Immunodeficiency Virus Infection and Associated with Progression of Carotid Artery Atherosclerosis. Clin. Infect. Dis. 2018, 67, 235–242.
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.-P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine Is an Endothelium-Derived Relaxing Factor Produced during Inflammation. Nat. Med. 2010, 16, 279–285.
- Joaquim, H.P.G.; Costa, A.C.; Gattaz, W.F.; Talib, L.L. Kynurenine Is Correlated with IL-1β in Plasma of Schizophrenia Patients. J. Neural Transm. 2018, 125, 869–873.
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957.
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of Functional GPR35 in Human INKT Cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425.
- Elizei, S.S.; Poormasjedi-Meibod, M.-S.; Wang, X.; Kheirandish, M.; Ghahary, A. Kynurenic Acid Downregulates IL-17/1L-23 Axis in Vitro. Mol. Cell. Biochem. 2017, 431, 55–65.
- Tiszlavicz, Z.; Tiszlavicz, Z.; Fülöp, F.; Vécsei, L.; Tápai, K.; Tápai, K.; Mándi, Y. Different Inhibitory Effects of Kynurenic Acid and a Novel Kynurenic Acid Analogue on Tumour Necrosis Factor-α (TNF-α) Production by Mononuclear Cells, HMGB1 Production by Monocytes and HNP1-3 Secretion by Neutrophils. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 383, 447–455.
- Walczak, K.; Dąbrowski, W.; Langner, E.; Zgrajka, W.; Piłat, J.; Piłat, J.; Rzeski, W.; Turski, W.A. Kynurenic Acid Synthesis and Kynurenine Aminotransferases Expression in Colon Derived Normal and Cancer Cells. Scand. J. Gastroenterol. 2011, 46, 903–912.
- Kaszaki, J.; Erces, D.; Varga, G.; Szabó, A.; Vécsei, L.; Boros, M. Kynurenines and Intestinal Neurotransmission: The Role of N-Methyl-D-Aspartate Receptors. J. Neural Transm. 2012, 119, 211–223.
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2017, 43, 764–777.
- Leipnitz, G.; Schumacher, C.; Dalcin, K.B.; Scussiato, K.; Solano, A.; Funchal, C.; Dutra-Filho, C.S.; Wyse, A.T.S.; Wannmacher, C.M.D.; Latini, A.; et al. In Vitro Evidence for an Antioxidant Role of 3-Hydroxykynurenine and 3-Hydroxyanthranilic Acid in the Brain. Neurochem. Int. 2007, 50, 83–94.
- Dorta, E.; Aspée, A.; Pino, E.; González, L.; Lissi, E.; López-Alarcón, C. Controversial Alkoxyl and Peroxyl Radical Scavenging Activity of the Tryptophan Metabolite 3-Hydroxy-Anthranilic Acid. Biomed. Pharmacother. 2017, 90, 332–338.
- Lee, S.-M.; Lee, Y.-S.; Choi, J.-H.; Park, S.-G.; Choi, I.-W.; Joo, Y.-D.; Lee, W.-S.; Lee, J.-N.; Choi, I.; Seo, S.-K. Tryptophan Metabolite 3-Hydroxyanthranilic Acid Selectively Induces Activated T Cell Death via Intracellular GSH Depletion. Immunol. Lett. 2010, 132, 53–60.
- Christen, S.; Peterhans, E.; Stocker, R. Antioxidant Activities of Some Tryptophan Metabolites: Possible Implication for Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 1990, 87, 2506–2510.
- Oxenkrug, G.; van der Hart, M.; Roeser, J.; Summergrad, P. Anthranilic Acid: A Potential Biomarker and Treatment Target for Schizophrenia. Ann. Psychiatry Ment. Health 2016, 4, 1059.
- Gęca, K.; Rawicz-Pruszyński, K.; Mlak, R.; Sadok, I.; Polkowski, W.P.; Staniszewska, M. Kynurenine and Anthranilic Acid in the Peritoneum Correlate With the Stage of Gastric Cancer Disease. Int. J. Tryptophan Res. 2022, 15, 1–10.
- Savitz, J.; Dantzer, R.; Wurfel, B.E.; Victor, T.A.; Ford, B.N.; Bodurka, J.; Bellgowan, P.S.F.; Teague, T.K.; Drevets, W.C. Neuroprotective Kynurenine Metabolite Indices Are Abnormally Reduced and Positively Associated with Hippocampal and Amygdalar Volume in Bipolar Disorder. Psychoneuroendocrinology 2015, 52, 200–211.
- Guillemin, G.J.; Brew, B.J.; Noonan, C.E.; Takikawa, O.; Cullen, K.M. Indoleamine 2,3 Dioxygenase and Quinolinic Acid Immunoreactivity in Alzheimer’s Disease Hippocampus. Neuropathol. Appl. Neurobiol. 2005, 31, 395–404.
- Guillemin, G.J.; Brew, B.J. Implications of the Kynurenine Pathway and Quinolinic Acid in Alzheimer’s Disease. Redox Rep. 2002, 7, 199–206.
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Copeland, C.S.; Neale, S.A.; Bruno, V.; Battaglia, G.; Salt, T.E.; Nicoletti, F. Cinnabarinic Acid and Xanthurenic Acid: Two Kynurenine Metabolites That Interact with Metabotropic Glutamate Receptors. Neuropharmacology 2017, 112, 365–372.
- Fazio, F.; Zappulla, C.; Notartomaso, S.; Busceti, C.; Bessede, A.; Scarselli, P.; Vacca, C.; Gargaro, M.; Volpi, C.; Allegrucci, M.; et al. Cinnabarinic Acid, an Endogenous Agonist of Type-4 Metabotropic Glutamate Receptor, Suppresses Experimental Autoimmune Encephalomyelitis in Mice. Neuropharmacology 2014, 81, 237–243.
- Curto, M.; Lionetto, L.; Fazio, F.; Corigliano, V.; Comparelli, A.; Ferracuti, S.; Simmaco, M.; Nicoletti, F.; Baldessarini, R.J. Serum Xanthurenic Acid Levels: Reduced in Subjects at Ultra High Risk for Psychosis. Schizophr. Res. 2019, 208, 465–466.
- Göçenoğlu, A.; Pazarlioglu, N. Cinnabarinic Acid: Enhanced Production from Pycnoporus Cinnabarinus, Characterization, Structural and Functional Properties. Hacet. J. Biol. Chem. 2014, 42, 281–290.
- Li, K.; Horanyi, P.S.; Collins, R.; Phillips, R.S.; Eriksson, K.-E.L. Investigation of the Role of 3-Hydroxyanthranilic Acid in the Degradation of Lignin by White-Rot Fungus Pycnoporus Cinnabarinus. Enzyme Microb. Technol. 2001, 28, 301–307.
- Waghmare, M.D.; Wasewar, K.L.; Sonawane, S.S.; Shende, D.Z. Reactive Extraction of Picolinic and Nicotinic Acid by Natural Non-Toxic Solvent. Sep. Purif. Technol. 2013, 120, 296–303.
- Senol, A. Influence of Conventional Diluents on Amine Extraction of Picolinic Acid. Sep. Purif. Technol. 2005, 43, 49–57.
This entry is offline, you can click here to edit this entry!
