Since Joseph Altman's groundbreaking research revealing neurogenesis in the adult rat hippocampus, the field has witnessed an exponential growth in publications. Researchers know that the adult hippocampus harbors a pool of adult neural stem cells (NSCs) driving life-long neurogenesis and plasticity. Aging significantly influences NSC functions, leading to a diminished capacity for generating new neurons and contributing to the gradual deterioration of hippocampus-related cognitive functions. Although the mechanisms underlying this age-related decline are only partially understood, factors such as increased NSC quiescence, altered differentiation patterns and NSC exhaustion have been linked to it.
- aging
- adult hippocampal neurogenesis
- adult neural stem cells
1. Introduction
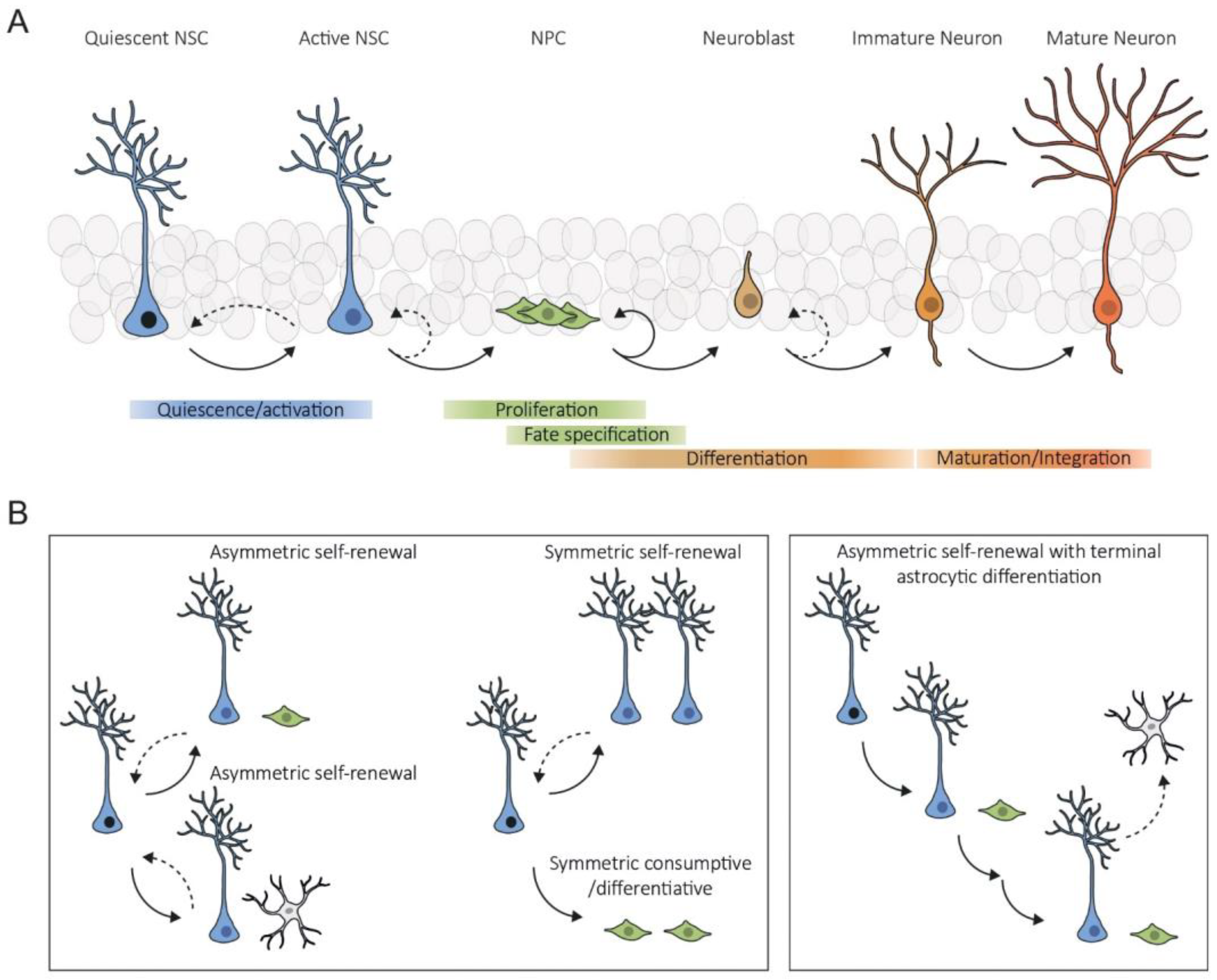
2. Intrinsic Mechanisms
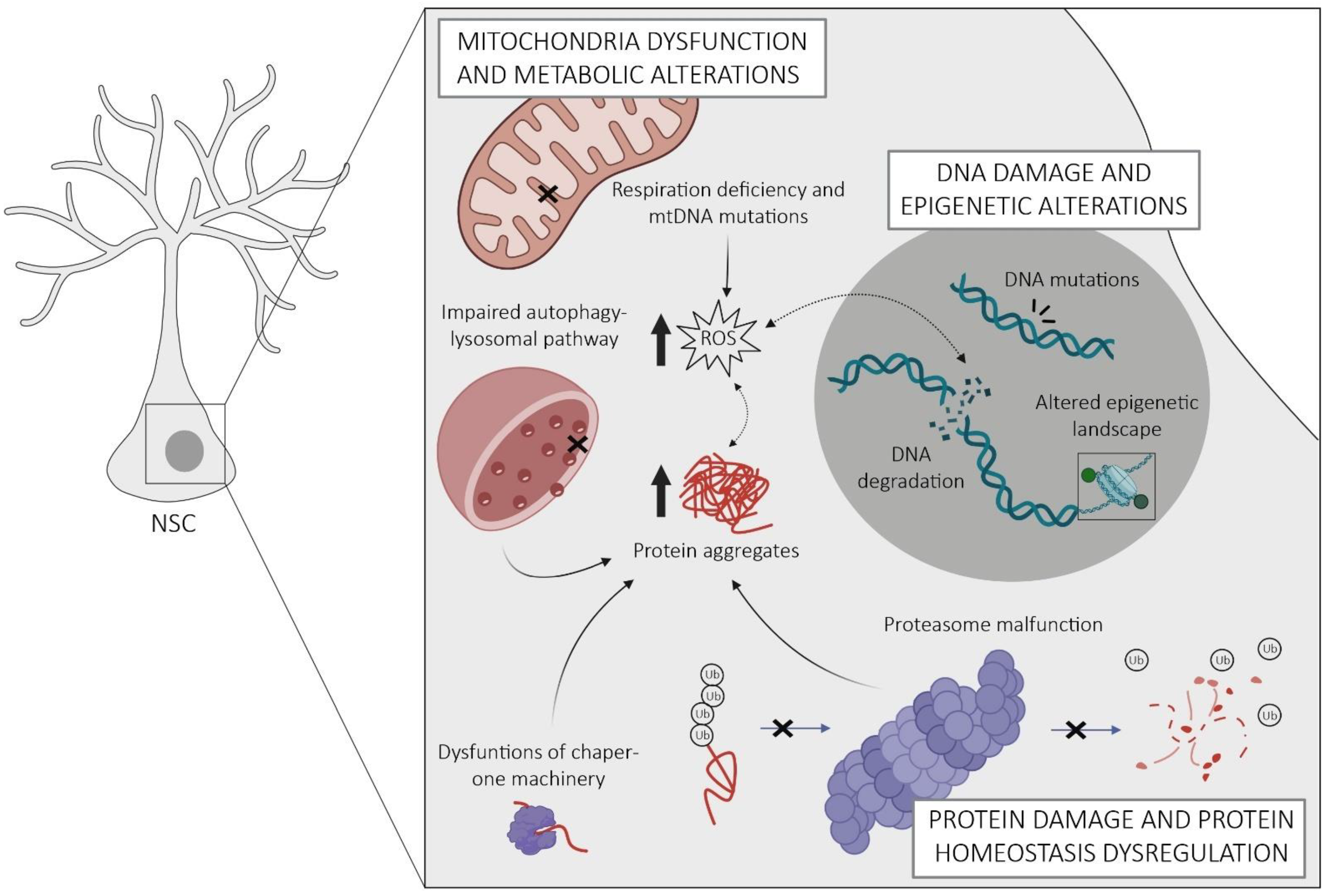
2.1. Protein Homeostasis Dysregulation
2.2. Mitochondria Dysfunction and Metabolic Alterations
2.3. DNA Damage and Epigenetic Alterations
2.4. Cellular Senescence
This entry is adapted from the peer-reviewed paper 10.3390/cells12162086
References
- Altman, J.; Das, G.D. Autoradiographic and Histological Evidence of Postnatal Hippocampal Neurogenesis in Rats. J. Comp. Neurol. 1965, 124, 319–335.
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcı, J.M.; Alvarez-buylla, A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain. Cell 1999, 97, 703–716.
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The Adult Rat Hippocampus Contains Primordial Neural Stem Cells. Mol. Cell. Neurosci. 1997, 8, 389–404.
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In Vivo Clonal Analysis Reveals Self-Renewing and Multipotent Adult Neural Stem Cell Characteristics. Cell 2011, 145, 1142–1155.
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-Coupled Astrocytic Differentiation and Age-Related Depletion of Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2011, 8, 566–579.
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jörg, D.J.; Carta, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live Imaging of Neurogenesis in the Adult Mouse Hippocampus. Science 2018, 359, 658–662.
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914.
- Ibrayeva, A.; Bay, M.; Pu, E.; Jörg, D.J.; Peng, L.; Jun, H.; Zhang, N.; Aaron, D.; Lin, C.; Resler, G.; et al. Early Stem Cell Aging in the Mature Brain. Cell Stem Cell 2021, 28, 955–966.e7.
- Lazutkin, A.; Podgorny, O.; Enikolopov, G. Modes of Division and Differentiation of Neural Stem Cells. Behav. Brain Res. 2019, 374, 112118.
- Urbán, N.; Van Den Berg, D.L.C.; Forget, A.; Andersen, J.; Demmers, J.A.A.; Hunt, C.; Ayrault, O.; Guillemot, F. Return to Quiescence of Mouse Neural Stem Cells by Degradation of a Proactivation Protein. Science 2016, 353, 292–295.
- Harris, L.; Rigo, P.; Stiehl, T.; Gaber, Z.B.; Austin, S.H.L.; Masdeu, M.d.M.; Edwards, A.; Urbán, N.; Marciniak-Czochra, A.; Guillemot, F. Coordinated Changes in Cellular Behavior Ensure the Lifelong Maintenance of the Hippocampal Stem Cell Population. Cell Stem Cell 2021, 28, 863–876.e6.
- Bast, L.; Calzolari, F.; Strasser, M.K.; Hasenauer, J.; Theis, F.J.; Ninkovic, J.; Marr, C. Increasing Neural Stem Cell Division Asymmetry and Quiescence Are Predicted to Contribute to the Age-Related Decline in Neurogenesis. Cell Rep. 2018, 25, 3231–3240.e8.
- Bottes, S.; Jaeger, B.N.; Pilz, G.A.; Jörg, D.J.; Cole, J.D.; Kruse, M.; Harris, L.; Korobeynyk, V.I.; Mallona, I.; Helmchen, F.; et al. Long-Term Self-Renewing Stem Cells in the Adult Mouse Hippocampus Identified by Intravital Imaging. Nat. Neurosci. 2021, 24, 225–233.
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848.
- Matsubara, S.; Matsuda, T.; Nakashima, K. Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors. Cells 2021, 10, 1145.
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the Dentate Gyrus of the Adult Rat: Age-Related Decrease of Neuronal Progenitor Proliferation. J. Neurosci. 1996, 16, 2027–2033.
- Bizon, J.L.; Gallagher, M. Production of New Cells in the Rat Dentate Gyrus over the Lifespan: Relation to Cognitive Decline. Eur. J. Neurosci. 2003, 18, 215–219.
- Klempin, F.; Kempermann, G. Adult Hippocampal Neurogenesis and Aging. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 271–280.
- Jessberger, S.; Toni, N.; Clemenson, G.D.; Ray, J.; Gage, F.H. Directed Differentiation of Hippocampal Stem/Progenitor Cells in the Adult Brain. Nat. Neurosci. 2008, 11, 888–893.
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult Hippocampal Neurogenesis Is Abundant in Neurologically Healthy Subjects and Drops Sharply in Patients with Alzheimer’s Disease. Nat. Med. 2019, 25, 554–560.
- Hattiangady, B.; Shetty, A.K. Aging Does Not Alter the Number or Phenotype of Putative Stem/Progenitor Cells in the Neurogenic Region of the Hippocampus. Neurobiol. Aging 2008, 29, 129–147.
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Götz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and Active Hippocampal Neural Stem Cells with Distinct Morphologies Respond Selectively to Physiological and Pathological Stimuli and Aging. Cell Stem Cell 2010, 6, 445–456.
- Wu, Y.; Bottes, S.; Fisch, R.; Zehnder, C.; Cole, J.D.; Pilz, G.A.; Helmchen, F.; Simons, B.D.; Jessberger, S. Chronic in Vivo Imaging Defines Age-Dependent Alterations of Neurogenesis in the Mouse Hippocampus. Nat. Aging 2023, 3, 380–390.
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and Function of Adult Neurogenesis: From Genes to Cognition. Physiol. Rev. 2014, 94, 991–1026.
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The Ageing Systemic Milieu Negatively Regulates Neurogenesis and Cognitive Function. Nature 2011, 477, 90–96.
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302.
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223.
- Saez, I.; Vilchez, D. The Mechanistic Links Between Proteasome Activity, Aging and Agerelated Diseases. Curr. Genom. 2014, 15, 38–51.
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332.
- Finley, D. Recognition and Processing of Ubiquitin-Protein Conjugates by the Proteasome. Annu. Rev. Biochem. 2009, 78, 477–513.
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650.
- Leeman, D.S.; Hebestreit, K.; Ruetz, T.; Webb, A.E.; McKay, A.; Pollina, E.A.; Dulken, B.W.; Zhao, X.; Yeo, R.W.; Ho, T.T.; et al. Lysosome Activation Clears Aggregates and Enhances Quiescent Neural Stem Cell Activation during Aging. Science 2018, 359, 1277–1283.
- Kobayashi, T.; Piao, W.; Takamura, T.; Kori, H.; Miyachi, H.; Kitano, S.; Iwamoto, Y.; Yamada, M.; Imayoshi, I.; Shioda, S.; et al. Enhanced Lysosomal Degradation Maintains the Quiescent State of Neural Stem Cells. Nat. Commun. 2019, 10, 2–4.
- Vonk, W.I.M.; Rainbolt, T.K.; Dolan, P.T.; Webb, A.E.; Brunet, A.; Frydman, J.; Vonk, W.I.M.; Rainbolt, T.K.; Dolan, P.T.; Webb, A.E.; et al. Differentiation Drives Widespread Rewiring of the Neural Stem Cell Chaperone Network Differentiation Drives Widespread Rewiring of the Neural Stem Cell Chaperone Network. Mol. Cell 2020, 78, 329–345.e9.
- Audesse, A.J.; Webb, A.E. Mechanisms of Enhanced Quiescence in Neural Stem Cell Aging. Mech. Ageing Dev. 2020, 191, 111323.
- Webb, A.E.; Brunet, A. FOXO Transcription Factors: Key Regulators of Cellular Quality Control. Trends Biochem. Sci. 2014, 39, 159–169.
- Schäffner, I.; Minakaki, G.; Khan, M.A.; Balta, E.A.; Schlötzer-Schrehardt, U.; Schwarz, T.J.; Beckervordersandforth, R.; Winner, B.; Webb, A.E.; DePinho, R.A.; et al. FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis. Neuron 2018, 99, 1188–1203.e6.
- Moore, D.L.; Pilz, G.A.; Araúzo-Bravo, M.J.; Barral, Y.; Jessberger, S. A Mechanism for the Segregation of Age in Mammalian Neural Stem Cells. Science 2015, 349, 1334–1338.
- Bedrosian, T.A.; Houtman, J.; Eguiguren, J.S.; Ghassemzadeh, S.; Rund, N.; Novaresi, N.M.; Hu, L.; Parylak, S.L.; Denli, A.M.; Randolph-Moore, L.; et al. Lamin B1 Decline Underlies Age-related Loss of Adult Hippocampal Neurogenesis. EMBO J. 2021, 40, e105819.
- Fatt, M.P.; Tran, L.M.; Vetere, G.; Storer, M.A.; Simonetta, J.V.; Miller, F.D.; Frankland, P.W.; Kaplan, D.R. Restoration of Hippocampal Neural Precursor Function by Ablation of Senescent Cells in the Aging Stem Cell Niche. Stem Cell Rep. 2022, 17, 259–275.
- Mahajani, S.; Giacomini, C.; Marinaro, F.; De Pietri Tonelli, D.; Contestabile, A.; Gasparini, L. Lamin B1 Levels Modulate Differentiation into Neurons during Embryonic Corticogenesis. Sci. Rep. 2017, 7, 4897.
- bin Imtiaz, M.K.; Jaeger, B.N.; Bottes, S.; Machado, R.A.C.; Vidmar, M.; Moore, D.L.; Jessberger, S. Declining Lamin B1 Expression Mediates Age-Dependent Decreases of Hippocampal Stem Cell Activity. Cell Stem Cell 2021, 28, 967–977.e8.
- Vahabikashi, A.; Adam, S.A.; Medalia, O.; Goldman, R.D. Nuclear Lamins: Structure and Function in Mechanobiology. APL Bioeng. 2022, 6, 011503.
- Beckervordersandforth, R.; Ebert, B.; Schäffner, I.; Moss, J.; Fiebig, C.; Shin, J.; Moore, D.L.; Ghosh, L.; Trinchero, M.F.; Stockburger, C.; et al. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 2017, 93, 560–573.e6.
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.L.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades Underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372.
- Petrelli, F.; Scandella, V.; Montessuit, S.; Zamboni, N.; Martinou, J.C.; Knobloch, M. Mitochondrial Pyruvate Metabolism Regulates the Activation of Quiescent Adult Neural Stem Cells. Sci. Adv. 2023, 9, eadd5220.
- Wani, G.A.; Sprenger, H.G.; Ndoci, K.; Chandragiri, S.; Acton, R.J.; Schatton, D.; Kochan, S.M.V.; Sakthivelu, V.; Jevtic, M.; Seeger, J.M.; et al. Metabolic Control of Adult Neural Stem Cell Self-Renewal by the Mitochondrial Protease YME1L. Cell Rep. 2022, 38, 110370.
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247.
- Etchegaray, J.P.; Mostoslavsky, R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol. Cell 2016, 62, 695–711.
- Wang, W.; Esbensen, Y.; Kunke, D.; Suganthan, R.; Rachek, L.; Bjørås, M.; Eide, L. Mitochondrial DNA Damage Level Determines Neural Stem Cell Differentiation Fate. J. Neurosci. 2011, 31, 9746–9751.
- Ma, C.Y.; Yao, M.J.; Zhai, Q.W.; Jiao, J.W.; Yuan, X.B.; Poo, M.M. SIRT1 Suppresses Self-Renewal of Adult Hippocampal Neural Stem Cells. Development 2014, 141, 4697–4709.
- Ortiz-González, X.R. Mitochondrial Dysfunction: A Common Denominator in Neurodevelopmental Disorders? Dev. Neurosci. 2021, 43, 222–229.
- Cinat, D.; Coppes, R.P.; Barazzuol, L. DNA Damage-Induced Inflammatory Microenvironment and Adult Stem Cell Response. Front. Cell Dev. Biol. 2021, 9, 2497.
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as Central Regulators of Neural Stem Cell Fate and Cognitive Function. Nat. Rev. Neurosci. 2019, 20, 34–48.
- Zhang, H.; Menzies, K.J.; Auwerx, J. The Role of Mitochondria in Stem Cell Fate and Aging. Dev. 2018, 145, dev143420.
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666.
- Siqueira, I.R.; Fochesatto, C.; De Andrade, A.; Santos, M.; Hagen, M.; Bello-Klein, A.; Netto, C.A. Total Antioxidant Capacity Is Impaired in Different Structures from Aged Rat Brain. Int. J. Dev. Neurosci. 2005, 23, 663–671.
- Raber, J.; Villasana, L.; Rosenberg, J.; Zou, Y.; Huang, T.T.; Fike, J.R. Irradiation Enhances Hippocampus-Dependent Cognition in Mice Deficient in Extracellular Superoxide Dismutase. Hippocampus 2011, 21, 72–80.
- Knobloch, M.; Braun, S.M.G.; Zurkirchen, L.; Von Schoultz, C.; Zamboni, N.; Araúzo-Bravo, M.J.; Kovacs, W.J.; Karalay, Ö.; Suter, U.; MacHado, R.A.C.; et al. Metabolic Control of Adult Neural Stem Cell Activity by Fasn-Dependent Lipogenesis. Nature 2013, 493, 226–230.
- Knobloch, M.; Pilz, G.A.; Ghesquière, B.; Kovacs, W.J.; Wegleiter, T.; Moore, D.L.; Hruzova, M.; Zamboni, N.; Carmeliet, P.; Jessberger, S. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 2017, 20, 2144–2155.
- Stoll, E.A.; Makin, R.; Sweet, I.R.; Trevelyan, A.J.; Miwa, S.; Horner, P.J.; Turnbull, D.M. Neural Stem Cells in the Adult Subventricular Zone Oxidize Fatty Acids to Produce Energy and Support Neurogenic Activity. Stem Cells 2015, 33, 2306–2319.
- Sênos Demarco, R.; Clémot, M.; Jones, D.L. The Impact of Ageing on Lipid-Mediated Regulation of Adult Stem Cell Behavior and Tissue Homeostasis. Mech. Aging Dev. 2020, 189, 111278.
- Martins, R.; Lithgow, G.J.; Link, W. Long Live FOXO: Unraveling the Role of FOXO Proteins in Aging and Longevity. Aging Cell 2016, 15, 196–207.
- Chaker, Z.; Aïd, S.; Berry, H.; Holzenberger, M. Suppression of IGF-I Signals in Neural Stem Cells Enhances Neurogenesis and Olfactory Function during Aging. Aging Cell 2015, 14, 847–856.
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet That Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99.
- Apple, D.M.; Mahesula, S.; Fonseca, R.S.; Zhu, C.; Kokovay, E. Calorie Restriction Protects Neural Stem Cells from Age-Related Deficits in the Subventricular Zone. Aging 2019, 11, 115–126.
- Fusco, S.; Leone, L.; Barbati, S.A.; Samengo, D.; Piacentini, R.; Maulucci, G.; Toietta, G.; Spinelli, M.; McBurney, M.; Pani, G.; et al. A CREB-Sirt1-Hes1 Circuitry Mediates Neural Stem Cell Response to Glucose Availability. Cell Rep. 2016, 14, 1195–1205.
- Amiri, A.; Cho, W.; Zhou, J.; Birnbaum, S.G.; Sinton, C.M.; McKay, R.M.; Parada, L.F. Pten Deletion in Adult Hippocampal Neural Stem/Progenitor Cells Causes Cellular Abnormalities and Alters Neurogenesis. J. Neurosci. 2012, 32, 5880–5890.
- Sahara, S.; Yanagawa, Y.; O’Leary, D.D.M.; Stevens, C.F. The Fraction of Cortical GABAergic Neurons Is Constant from near the Start of Cortical Neurogenesis to Adulthood. J. Neurosci. 2012, 32, 4755–4761.
- Imai, S.-I.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471.
- Mani, C.; Reddy, P.H.; Palle, K. DNA Repair Fidelity in Stem Cell Maintenance, Health, and Disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165444.
- Singh, R.P.; Shiue, K.; Schomberg, D.; Zhou, F.C. Cellular Epigenetic Modifications of Neural Stem Cell Differentiation. Cell Transplant. 2009, 18, 1197–1211.
- Maybury-Lewis, S.Y.; Brown, A.K.; Yeary, M.; Sloutskin, A.; Dhakal, S.; Juven-Gershon, T.; Webb, A.E. Changing and Stable Chromatin Accessibility Supports Transcriptional Overhaul during Neural Stem Cell Activation and Is Altered with Age. Aging Cell 2021, 20, e13499.
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The Central Role of DNA Damage in the Ageing Process. Nature 2021, 592, 695–703.
- Zocher, S.; Toda, T. Epigenetic Aging in Adult Neurogenesis. Hippocampus 2023, 33, 347–359.
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA Damage: Cause, Effect or Both. Nat. Rev. Rheumatol. 2023, 19, 200–211.
- Behrens, A.; Van Deursen, J.M.; Rudolph, K.L.; Schumacher, B. Impact of Genomic Damage and Ageing on Stem Cell Function. Nat. Cell Biol. 2014, 16, 201–207.
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA Damage and Repair in Age-Related Inflammation. Nat. Rev. Immunol. 2023, 23, 75–89.
- Bailey, K.J.; Maslov, A.Y.; Pruitt, S.C. Accumulation of Mutations and Somatic Selection in Aging Neural Stem/Progenitor Cells. Aging Cell 2004, 3, 391–397.
- Schneider, L.; Pellegatta, S.; Favaro, R.; Pisati, F.; Roncaglia, P.; Testa, G.; Nicolis, S.K.; Finocchiaro, G.; D’Adda Di Fagagna, F. DNA Damage in Mammalian Neural Stem Cells Leads to Astrocytic Differentiation Mediated by BMP2 Signaling through JAK-STAT. Stem Cell Rep. 2013, 1, 123–138.
- Barazzuol, L.; Ju, L.; Jeggo, P.A. A Coordinated DNA Damage Response Promotes Adult Quiescent Neural Stem Cell Activation. PLoS Biol. 2017, 15, e2001264.
- Daynac, M.; Chicheportiche, A.; Pineda, J.R.; Gauthier, L.R.; Boussin, F.D.; Mouthon, M.A. Quiescent Neural Stem Cells Exit Dormancy upon Alteration of GABAAR Signaling Following Radiation Damage. Stem Cell Res. 2013, 11, 516–528.
- Ben Abdallah, N.M.B.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.P. Early Age-Related Changes in Adult Hippocampal Neurogenesis in C57 Mice. Neurobiol. Aging 2010, 31, 151–161.
- Walter, D.; Lier, A.; Geiselhart, A.; Thalheimer, F.B.; Huntscha, S.; Sobotta, M.C.; Moehrle, B.; Brocks, D.; Bayindir, I.; Kaschutnig, P.; et al. Exit from Dormancy Provokes DNA-Damage-Induced Attrition in Haematopoietic Stem Cells. Nature 2015, 520, 549–552.
- Hellström, N.A.K.; Björk-Eriksson, T.; Blomgren, K.; Kuhn, H.G. Differential Recovery of Neural Stem Cells in the Subventricular Zone and Dentate Gyrus After Ionizing Radiation. Stem Cells 2009, 27, 634–641.
- Kalamakis, G.; Brüne, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catalá-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e14.
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The Maintenance of Mitochondrial DNA Integrity—Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641.
- Aboelnour, E.; Bonev, B. Decoding the Organization, Dynamics, and Function of the 4D Genome. Dev. Cell 2021, 56, 1562–1573.
- Zocher, S.; Overall, R.W.; Lesche, M.; Dahl, A.; Kempermann, G. Environmental Enrichment Preserves a Young DNA Methylation Landscape in the Aged Mouse Hippocampus. Nat. Commun. 2021, 12, 3892.
- Gontier, G.; Iyer, M.; Shea, J.M.; Bieri, G.; Wheatley, E.G.; Ramalho-Santos, M.; Villeda, S.A. Tet2 Rescues Age-Related Regenerative Decline and Enhances Cognitive Function in the Adult Mouse Brain. Cell Rep. 2018, 22, 1974–1981.
- Jobe, E.M.; Gao, Y.; Eisinger, B.E.; Mladucky, J.K.; Giuliani, C.C.; Kelnhofer, L.E.; Zhao, X. Methyl-Cpg-Binding Protein MBD1 Regulates Neuronal Lineage Commitment through Maintaining Adult Neural Stem Cell Identity. J. Neurosci. 2017, 37, 523–536.
- Li, X.; Barkho, B.Z.; Luo, Y.; Smrt, R.D.; Santistevan, N.J.; Liu, C.; Kuwabara, T.; Gage, F.H.; Zhao, X. Epigenetic Regulation of the Stem Cell Mitogen Fgf-2 by Mbd1 in Adult Neural Stem/Progenitor Cells. J. Biol. Chem. 2008, 283, 27644–27652.
- Iwafuchi-Doi, M.; Zaret, K.S. Pioneer Transcription Factors in Cell Reprogramming. Genes Dev. 2014, 28, 2679–2692.
- Zaret, K.S. Pioneer Transcription Factors Initiating Gene Network Changes. Annu. Rev. Genet. 2020, 54, 367–385.
- Raposo, A.A.S.F.; Vasconcelos, F.F.; Drechsel, D.; Marie, C.; Johnston, C.; Dolle, D.; Bithell, A.; Gillotin, S.; van den Berg, D.L.C.; Ettwiller, L.; et al. Ascl1 Coordinately Regulates Gene Expression and the Chromatin Landscape during Neurogenesis. Cell Rep. 2015, 10, 1544–1556.
- Blomfield, I.M.; Rocamonde, B.; del Mar Masdeu, M.; Mulugeta, E.; Vaga, S.; van den Berg, D.L.C.; Huillard, E.; Guillemot, F.; Urbán, N. Id4 Promotes the Elimination of the Pro-Activation Factor Ascl1 to Maintain Quiescence of Adult Hippocampal Stem Cells. Elife 2019, 8, e48561.
- Andersen, J.; Urbán, N.; Achimastou, A.; Ito, A.; Simic, M.; Ullom, K.; Martynoga, B.; Lebel, M.; Göritz, C.; Frisén, J.; et al. A Transcriptional Mechanism Integrating Inputs from Extracellular Signals to Activate Hippocampal Stem Cells. Neuron 2014, 83, 1085–1097.
- Ferri, A.L.M.; Cavallaro, M.; Braida, D.; Di Cristofano, A.; Canta, A.; Vezzani, A.; Ottolenghi, S.; Pandolfi, P.P.; Sala, M.; DeBiasi, S.; et al. Sox2 Deficiency Causes Neurodegeneration and Impaired Neurogenesis in the Adult Mouse Brain. Development 2004, 131, 3805–3819.
- Bertolini, J.A.; Favaro, R.; Zhu, Y.; Pagin, M.; Ngan, C.Y.; Wong, C.H.; Tjong, H.; Vermunt, M.W.; Martynoga, B.; Barone, C.; et al. Mapping the Global Chromatin Connectivity Network for Sox2 Function in Neural Stem Cell Maintenance. Cell Stem Cell 2019, 24, 462–476.e6.
- Cimadamore, F.; Amador-Arjona, A.; Chen, C.; Huang, C.T.; Terskikh, A.V. SOX2-LIN28/Let-7 Pathway Regulates Proliferation and Neurogenesis in Neural Precursors. Proc. Natl. Acad. Sci. USA 2013, 110, 3017–3026.
- Amador-Arjona, A.; Cimadamore, F.; Huang, C.T.; Wright, R.; Lewis, S.; Gage, F.H.; Terskikh, A.V. SOX2 Primes the Epigenetic Landscape in Neural Precursors Enabling Proper Gene Activation during Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E1936–E1945.
- Toda, T.; Hsu, J.Y.; Linker, S.B.; Hu, L.; Schafer, S.T.; Mertens, J.; Jacinto, F.V.; Hetzer, M.W.; Gage, F.H. Nup153 Interacts with Sox2 to Enable Bimodal Gene Regulation and Maintenance of Neural Progenitor Cells. Cell Stem Cell 2017, 21, 618–634.e7.
- Carrasco-Garcia, E.; Moreno-Cugnon, L.; Garcia, I.; Borras, C.; Revuelta, M.; Izeta, A.; Lopez-Lluch, G.; de Pancorbo, M.M.; Vergara, I.; Vina, J.; et al. SOX2 Expression Diminishes with Ageing in Several Tissues in Mice and Humans. Mech. Ageing Dev. 2019, 177, 30–36.
- Mestres, I.; Houtman, J.; Calegari, F.; Toda, T. A Nuclear Belt Fastens on Neural Cell Fate. Cells 2022, 11, 1761.
- Peric-Hupkes, D.; Meuleman, W.; Pagie, L.; Bruggeman, S.W.M.; Solovei, I.; Brugman, W.; Gräf, S.; Flicek, P.; Kerkhoven, R.M.; van Lohuizen, M.; et al. Molecular Maps of the Reorganization of Genome-Nuclear Lamina Interactions during Differentiation. Mol. Cell 2010, 38, 603–613.
- Chang, L.; Li, M.; Shao, S.; Li, C.; Ai, S.; Xue, B.; Hou, Y.; Zhang, Y.; Li, R.; Fan, X.; et al. Nuclear Peripheral Chromatin-Lamin B1 Interaction Is Required for Global Integrity of Chromatin Architecture and Dynamics in Human Cells. Protein Cell 2022, 13, 258–280.
- Matias, I.; Diniz, L.P.; Damico, I.V.; Araujo, A.P.B.; Neves, L.d.S.; Vargas, G.; Leite, R.E.P.; Suemoto, C.K.; Nitrini, R.; Jacob-Filho, W.; et al. Loss of Lamin-B1 and Defective Nuclear Morphology Are Hallmarks of Astrocyte Senescence in Vitro and in the Aging Human Hippocampus. Aging Cell 2022, 21, e13521.
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of Epigenetic Information as a Cause of Mammalian Aging. Cell 2023, 186, 305–326.e27.
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453.
- Si, Z.; Sun, L.; Wang, X. Evidence and Perspectives of Cell Senescence in Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111327.
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A Biomarker That Identifies Senescent Human Cells in Culture and in Aging Skin in Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367.
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic Ras Provokes Premature Cell Senescence Associated with Accumulation of P53 and P16(INK4a). Cell 1997, 88, 593–602.
- Sherr, C.J.; Roberts, J.M. CDK Inhibitors: Positive and Negative Regulators of G1-Phase Progression. Genes Dev. 1999, 13, 1501–1512.
- Ahlenius, H.; Visan, V.; Kokaia, M.; Lindvall, O.; Kokaia, Z. Neural Stem and Progenitor Cells Retain Their Potential for Proliferation and Differentiation into Functional Neurons despite Lower Number in Aged Brain. J. Neurosci. 2009, 29, 4408–4419.
- Urbach, A.; Witte, O.W. Divide or Commit—Revisiting the Role of Cell Cycle Regulators in Adult Hippocampal Neurogenesis. Front. Cell Dev. Biol. 2019, 7, 55.
- Molofsky, A.V.; Slutsky, S.G.; Joseph, N.M.; He, S.; Pardal, R.; Krishnamurthy, J.; Sharpless, N.E.; Morrison, S.J. Increasing P16INK4a Expression Decreases Forebrain Progenitors and Neurogenesis during Ageing. Nature 2006, 443, 448–452.
- Micheli, L.; D’Andrea, G.; Ceccarelli, M.; Ferri, A.; Scardigli, R.; Tirone, F. P16Ink4a Prevents the Activation of Aged Quiescent Dentate Gyrus Stem Cells by Physical Exercise. Front. Cell. Neurosci. 2019, 13, 10.
- Soriano-Cantón, R.; Perez-Villalba, A.; Morante-Redolat, J.M.; Marqués-Torrejón, M.Á.; Pallás, M.; Pérez-Sánchez, F.; Fariñas, I. Regulation of the P19Arf/P53 Pathway by Histone Acetylation Underlies Neural Stem Cell Behavior in Senescence-Prone SAMP8 Mice. Aging Cell 2015, 14, 453–462.
- Van Deursen, J.M. The Role of Senescent Cells in Ageing. Nature 2014, 509, 439–446.
- Freund, A.; Laberge, R.M.; Demaria, M.; Campisi, J. Lamin B1 Loss Is a Senescence-Associated Biomarker. Mol. Biol. Cell 2012, 23, 2066–2075.
- Gasperini, C.; Tuntevski, K.; Beatini, S.; Pelizzoli, R.; Lo Van, A.; Mangoni, D.; Cossu, R.M.; Pascarella, G.; Bianchini, P.; Bielefeld, P.; et al. Piwil2 (Mili) Sustains Neurogenesis and Prevents Cellular Senescence in the Postnatal Hippocampus. EMBO Rep. 2023, 24, e53801.
- Kaise, T.; Fukui, M.; Sueda, R.; Piao, W.; Yamada, M.; Kobayashi, T.; Imayoshi, I.; Kageyama, R. Functional Rejuvenation of Aged Neural Stem Cells by Plagl2 and Anti-Dyrk1a Activity. Genes Dev. 2022, 36, 23–37.
- Liu, Y.-D.; Zhu, Y.-T.; Shi, Y.-H.; Liu, X.-X.; Su, W.-R.; Zhuo, Y.-H. Deciphering Adult Neural Stem Cells with Single-Cell Sequencing. Stem Cells Dev. 2023, 32, 213–224.
