Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Fabry disease (FD) is a genetic lysosomal storage disease with frequent cardiovascular involvement, whose presence is a major determinant of adverse clinical outcomes. As a potentially treatable cause of left ventricular hypertrophy (LVH) and heart failure with preserved ejection fraction, the early recognition of FD is crucial to initiate enzyme replacement therapy and improve long-term prognosis. Multimodality imaging plays a central role in the evaluation of patients with FD and helps in the differential diagnosis of other conditions presenting with LVH.
- echocardiography
- CMR
- cardiovascular magnetic resonance
1. Introduction
Cardiac imaging has proved fundamental in each aspect of Fabry cardiomyopathy. Echocardiography offers several techniques for the evaluation of patients with fabry disease (FD), ranging from standard two-dimensional transthoracic echocardiography and conventional Doppler to tissue Doppler and speckle-tracking methods. CMR has gained a leading role in FD as it is the reference modality for wall thickness and chamber size assessment (not requiring geometrical assumptions and good acoustic windows), regional and global function of the myocardium, or non-invasive tissue characterization in a wide spectrum of cardiomyopathies. Cardiovascular magnetic resonance (CMR) has also provided significant insights into the mechanisms that lead from Gb3 deposition to inflammation and fibrosis. Early recognition of FD in the context of unexplained LVH is crucial to initiate enzyme replacement therapy (ERT) and limit the progression of the disease. CMR can unveil subclinical myocardial involvement, help to non-invasively differentiate FD from other diseases manifesting with LVH, mainly hypertrophic cardiomyopathy (HCM), and stage the progression of the disease. The role of cardiac imaging is of great importance, especially in patients with cardiac variant FD, as they may be lacking suggestive extra-cardiac involvement.
2. Echocardiography
Echocardiography is often the first imaging modality to be performed in suspected FD or to investigate otherwise unexplained ECG abnormalities, and it is also useful during the follow-up of affected patients. The hallmarks of FD are concentric LV remodeling or hypertrophy with the often-disproportionate hypertrophy of the papillary muscles; preserved systolic function, as measured by a left ventricular ejection fraction (LVEF) with an impaired myocardial strain; and right ventricular (RV) hypertrophy (Figure 1).
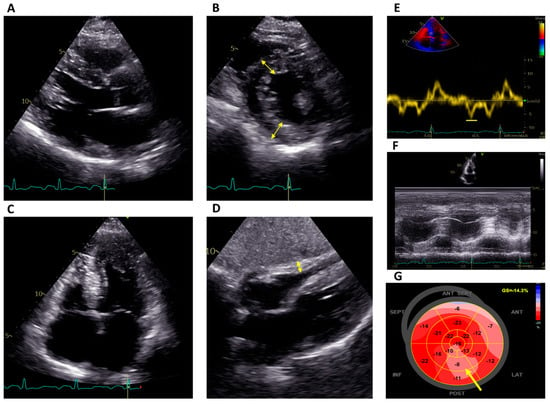
Figure 1. Echocardiographic features of a patient with Fabry disease on ERT. (A–C) Parasternal long-axis view, short-axis view at the level of the papillary muscles and four-chamber apical view, respectively, showing concentric left ventricular hypertrophy (double-edged arrows; septum 17 mm) with hypertrophy of the papillary muscles. (D) Subcostal four-chamber view demonstrating RV hypertrophy (double-edged arrow). (E) Reduced tissue Doppler septal strain e’ wave (6 cm/s). (F) Normal RV function (TAPSE 23 mm). (G) Reduced global longitudinal strain (−14.3%) with a regional reduction in the basal and mid inferolateral segments (arrow). ERT: Enzyme replacement therapy; RV: right ventricular.
LVH is not specific to FD; it is present in other more common conditions, and other patterns of hypertrophy have been described, such as asymmetrical septal or apical hypertrophy, or even outflow tract obstruction (as in HCM). LVH is more common in male patients with FD, increases with age, and is associated with symptoms [14]. RV hypertrophy is present in 31–71% patients and the degree thereof correlates with LVH, without sex differences. RV systolic dysfunction, though, is rare [15,16].
Global longitudinal strain (GLS), a reliable marker of LV systolic function in a broad spectrum of cardiomyopathies, is an early marker of FD cardiomyopathy, potentially useful for identifying gene carriers before LVH becomes manifest [17,18]. In a study, a reduction in longitudinal strain was present in the basal segments of all FD patients with LVH and 50% of those not yet showing LVH, while it was absent in all patients of the control group [19]. In another study, despite no difference in LVEF between FD patients and healthy subjects, GLS was impaired in the first group (−16.5 ± 3.8% vs. −20.2 ± 1.7%), especially in those with LVH. Additionally, the longitudinal strain was most reduced in the basal segments of the LV in patients with FD [20]. Interestingly, a lower longitudinal strain in the basal inferolateral segment showed a correlation with the extent of LGE when using CMR [21]. A regional longitudinal strain worse than 12.5% was a strong predictor of fibrosis in that segment, while a regional longitudinal strain better than 16.5% could reliably exclude fibrosis [21]. The loss of a base-to-apex circumferential strain gradient, defined as the peak gradient difference between the averaged basal and apical strains, has been proposed as a specific finding in FD cardiomyopathy, regardless of the presence of LVH [22].
Diastolic dysfunction is common among patients with LVH and is sometimes observed even before LVH develops. Reduced tissue Doppler velocities, higher E/e’ ratios, and shortened isovolumetric relaxation times are early markers of cardiac involvement [23,24,25]. A restrictive filling pattern is rarely present and is usually associated with advanced cardiomyopathy. Similarly, left atrial (LA) enlargement and reduced atrial compliance have been described early in the course of the disease, in the pre-hypertrophic phase [26].
The “binary sign”, defined as a hyperechogenic stripe in the LV myocardium adjacent to a relatively hypoechogenic region, once considered an almost pathognomonic sign of FD [27], has been reconsidered in recent years [28,29]. Other reported findings in FD are aortic root dilatation and the thickening of the mitral and aortic valves secondary to the deposition of Gb3, although moderate or greater regurgitation is rare [30].
3. Cardiovascular Magnetic Resonance
CMR allows us to precisely identify and quantify left and right ventricular hypertrophy, including papillary muscle hypertrophy, and assess its spatial distribution (Figure 2).
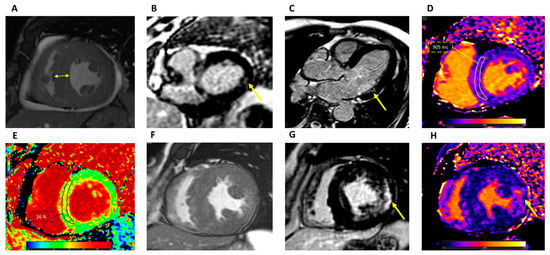
Figure 2. CMR findings in Fabry disease. (A) Cine short-axis view demonstrating concentric LV hypertrophy (double-edged arrow). LGE short-axis at the basal level (B) and three-chamber view (C) demonstrating intramural LGE in the basal inferolateral wall (arrow). (D) T1 mapping showing reduced intramyocardial T1 values: ROI 905 ± 35 ms at 1.5 T. (E) ECV map showing normal intramyocardial values: ROI 26 ± 3%. (F) Cine short-axis view demonstrating concentric LV hypertrophy and papillary muscles hypertrophy in a patient with advanced disease. (G) LGE short-axis view at the level of the papillary muscles showing extensive intramural LGE at the lateral segments and other foci in the septum and RV. (H) T1 mapping showing pseudo-normalization of T1 values (1021 ± 98 ms at 1.5 T) with areas demonstrating reduced values and areas, such as the one indicated by the arrow, corresponding to LGE, with focal elevation up to 1100 ms. CMR: cardiovascular magnetic resonance; LV: left ventricular; LGE: late gadolinium enhancement; ROI: region of interest; ECV: extracellular volume; RV: right ventricular.
CMR is more sensitive than echocardiography in detecting changes in LV mass [31,32]. LV mass is usually increased in FD patients; as discussed before, the contribution of LV papillary muscle mass to the total LV mass is disproportionately increased: from 8% in normal subjects to 20% in FD patients with LVH [33]. In a study, in patients with FD, papillary muscle hypertrophy was found even in the absence of LVH, supporting the notion that it is a helpful marker of FD [34]. The inclusion or exclusion of papillary muscles in the quantification of LV mass should always be specified in the CMR report, and appropriate reference values should be used.
LGE, representing areas of replacement fibrosis, is present in about 50% of patients [35]. The presence of LGE can aid in the diagnosis of FD, especially in the setting of LVH. LGE is initially focally distributed, and the most typical pattern of LGE distribution in the inferolateral basal-to-mid LV wall is mid-myocardial. As fibrosis develops, the thinning, hypokinesia, or akinesia of the same segments can sometimes be observed in advanced stages. However, other conditions, such as myocarditis, may also present with a similar LGE pattern. In patients with LVH with an atypical distribution for FD, LGE has been observed in the basal segment of the anterior septum and in the apical segments, resembling HCM. In patients with advanced disease, LGE tends to become extensive with a less specific appearance [36], and LVEF can be reduced.
A study by Niemann [37] found that the association between LGE and LVH varies depending on sex: in males, LVH precedes the development of fibrosis detectable through LGE imaging; on the contrary, in 23% of female patients, LGE was detectable even though LV wall thickness was normal, suggesting that the progression of the disease takes different pathways in males and females. LGE, thus, especially in female patients, is crucial for the detection of cardiac involvement and decisions on therapy initiation.
T1 mapping, a quantitative technique used to derive pixel-by-pixel T1 relaxation times of the myocardium, is particularly useful in FD. Likely due to the intramyocardial deposition of sphingolipids, native T1 values are lower than expected in other cardiomyopathies presenting with LVH, where native T1 is normal or, more frequently, increased, except for iron overload cardiomyopathy. Low T1 values can also testify to LV involvement before hypertrophy and fibrosis appear, allowing for an early diagnosis of cardiac involvement [38,39]. Low T1 values are present in 48% to 59% of patients without LVH, and an association has also been found with impaired LV-GLS [40]. A recent meta-analysis of 14 studies established that the weighted mean native T1 values were 984 ± 47 ms in FD patients and 1016 ± 26 ms in healthy subjects, with a pooled standardized mean difference of −2.38, and the degree of T1 shortening was influenced by the male sex and the presence of LVH [41]. As discussed before, sphingolipid deposition triggers inflammatory and profibrotic cellular pathways, which in turn tend to increase regional T1 values over time in areas of fibrosis (pseudo-normalization of T1 values); in the advanced stage of the disease, T1 values may be globally elevated due to diffuse fibrosis. Nonetheless, low T1 values in the context of LVH should prompt further evaluation for FD. On the other hand, normal native T1 values do not exclude FD cardiomyopathy, sometimes being noticed in untreated patients with mild LVH (especially females) or in advanced disease due to concomitant myocardial fibrosis. Interestingly, FD patients who have not yet developed LVH and detectable myocardial storage through T1 shortening still show lower T1 values, albeit in the normal range, compared to healthy controls, as well as subtly reduced LV-GLS, microvascular dysfunction and ECG abnormalities [42]. As such, the screening of FD gene carriers with GLS and T1 mapping seems a promising strategy to unmask the earliest signs of cardiac involvement.
Extracellular volume (ECV) is usually normal in FD except for areas with LGE, confirming that, at least in part, LVH is due to cell hypertrophy rather than extracellular matrix expansion [43].
T2 mapping shows elevated T2 values, both globally and in areas with LGE; this finding has been described [44] as a peculiar feature of FD ventricular hypertrophy, which can help in further differentiating sarcomeric HCM from FD phenocopies, thus supporting a pivotal role for inflammation in the pathogenesis of FD hypertrophy and disease progression toward myocardial replacement fibrosis.
Atrial function, expressed as an LA conduit strain and evaluated using feature tracking CMR, has been found to be already reduced in patients in the pre-hypertrophic phase, where only T1 mapping abnormalities were present [45]. This probably testifies to underlying atrial myopathy due to Gb3 deposition occurring earlier than LV remodeling.
The characteristic features of FD using echocardiography and CMR are summarized in Table 1.
Table 1. Fabry disease features assessable using echocardiography, CMR or both.
| Echocardiography | CMR |
|---|---|
| Concentric LV hypertrophy with disproportionate hypertrophy of the papillary muscles [33] | |
| Elevated LV mass [14] | |
| Right ventricular hypertrophy with normal systolic function [15,16] | |
| Reduced tissue Doppler strain [23] | Basal inferolateral LGE [35] |
| Reduced GLS [17,18] | Reduced global native T1 (may be normal or increased in advanced stages) [38,39] |
| Abnormal basal inferolateral regional strain [19] | Normal ECV (may be increased in advanced stages) [43] |
| Loss of basal-to-apex circumferential strain gradient [22] | Elevated T2 in the basal inferolateral wall [44] |
| LA dilation (not specific to FD) [26] | |
CMR: cardiovascular magnetic resonance; LV: left ventricular; LGE: late gadolinium enhancement; GLS: global longitudinal strain; ECV; extracellular volume; LA: left atrium.
4. Differential Diagnosis of Left Ventricular Hypertrophy
A hypertrophic phenotype may develop as an adaptive response to different stimuli, and CMR is key in the differential diagnosis of other diseases presenting with LVH, such as amyloidosis, HCM, aortic stenosis and hypertensive cardiomyopathy, or even LV hypertrophy in athletes, although a degree of overlap exists between these different conditions. FD is regarded as a phenocopy of HCM and, indeed, timely diagnosis is often missed as some patients with FD are misdiagnosed as having HCM and excluded from disease-modifying treatments. Accordingly, screening studies conducted in patients with HCM have found a prevalence of GLA gene mutations, especially those causing late-onset cardiac variants, of around 1% [46,47]. Similarly, in a series of patients undergoing surgical myectomy for LV outflow tract obstruction, a condition traditionally associated with HCM, a genetic analysis of the sample confirmed a GLA mutation in 1.3% of the patients [48]. These findings advocate for the systematic screening of FD in adult patients with the HCM phenotype, although it must be recognized that a subset of FD patients with late-onset variants have LVH that does not reach the 15 mm threshold for HCM diagnosis.
HCM sometimes can manifest with concentric hypertrophy, but the degree of hypertrophy is usually greater than with FD; in HCM, LGE and impaired regional strain are typically present in the most hypertrophied segments. In a study of 40 patients with LVH divided into 2 groups (FD and HCM) and matched for the degree of LVH and age, the FD group had lower LVEF, more reduced regional longitudinal strain in the inferolateral wall of the LV, and more impaired RV-free wall longitudinal strain, and the pattern of hypertrophy was more often concentric [49]. The finding of more profound subclinical RV impairment was confirmed in a recent study of 140 patients with FD or HCM and may be helpful in the differential diagnosis [50].
CMR-based studies showed that atrial remodeling, expressed as more pronounced LA dilatation and worse LA strain, is greater in HCM than in FD cardiomyopathy, with a good potential for distinguishing the two conditions [51]. Another study using 3T CMR found that a septal native T1 lower than 1220 ms distinguished patients with FD from HCM with an accuracy of 95%, providing incremental diagnostic value beyond age, sex, and conventional imaging features [52]. The association of myocardial hypertrophy with low native T1 values and elevated T2 values should be considered suggestive of FD-associated cardiac involvement (Table 2).
Table 2. Differential imaging features between Fabry disease and hypertrophic cardiomyopathy.
| Fabry Disease | Hypertrophic Cardiomyopathy | |
|---|---|---|
| Left ventricular hypertrophy | Usually concentric [49] | Usually asymmetrical, apical or segmental [53] |
| Papillary muscles | Disproportionately hypertrophied [33] | Sometimes apically displaced [54] |
| Late gadolinium enhancement | Mid-wall or subepicardial basal inferolateral [35] | Mid-wall in most hypertrophied segments and RV insertion points [53,55] |
| T1 mapping | Global native T1 reduced; normal or elevated in advanced disease [38,39] | Normal or elevated T1 values [52,56] |
| Extracellular volume | Generally normal [43] | Generally normal or elevated [57] |
| T2 mapping | Elevated values in the basal inferolateral wall [44] | Sometimes elevated values in severely hypertrophied segments [57] |
| Global longitudinal strain | Usually impaired in the basal inferolateral wall [19] | Impaired in areas of hypertrophy [58] |
RV: right ventricular.
In cardiac amyloidosis, instead, native T1 values and ECV are globally increased, and LGE is often present with a global subendocardial or transmural pattern. LGE is less helpful in aortic stenosis and hypertensive cardiomyopathy, but elevated T1 values coupled with a globally reduced longitudinal strain in AS and regionally at the level of the septum in hypertensive cardiomyopathy are valuable clues.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12227061
This entry is offline, you can click here to edit this entry!
