Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Silk secreted by Bombyx mori L. silkworm has become one of the most important biomaterials, due to its excellent biocompatibility, controllable biodegradability, superior processability, and unique mechanical properties. Silk fibroin and sericin, as the two components of silk, contain abundant polar functional groups, and thus can bind metal ions through electrostatic interaction and chelation.
- silk protein
- silk peptide
- metal ion
- mechanical property
- heavy metal-contaminated water remediation
1. Amino Acid Composition
Bombyx mori L. silk is composed of SF and sericin, featuring an elliptical cross-section and a diameter of approximately 30 μm (Figure 1A). The SF contains two SF fibers, each of which exhibits a triangular or semi-elliptical shape, with a diameter of roughly 10 μm. In silk, SF comprises 70–80%, while silk sericin accounts for about 20–30% [1][2][3].
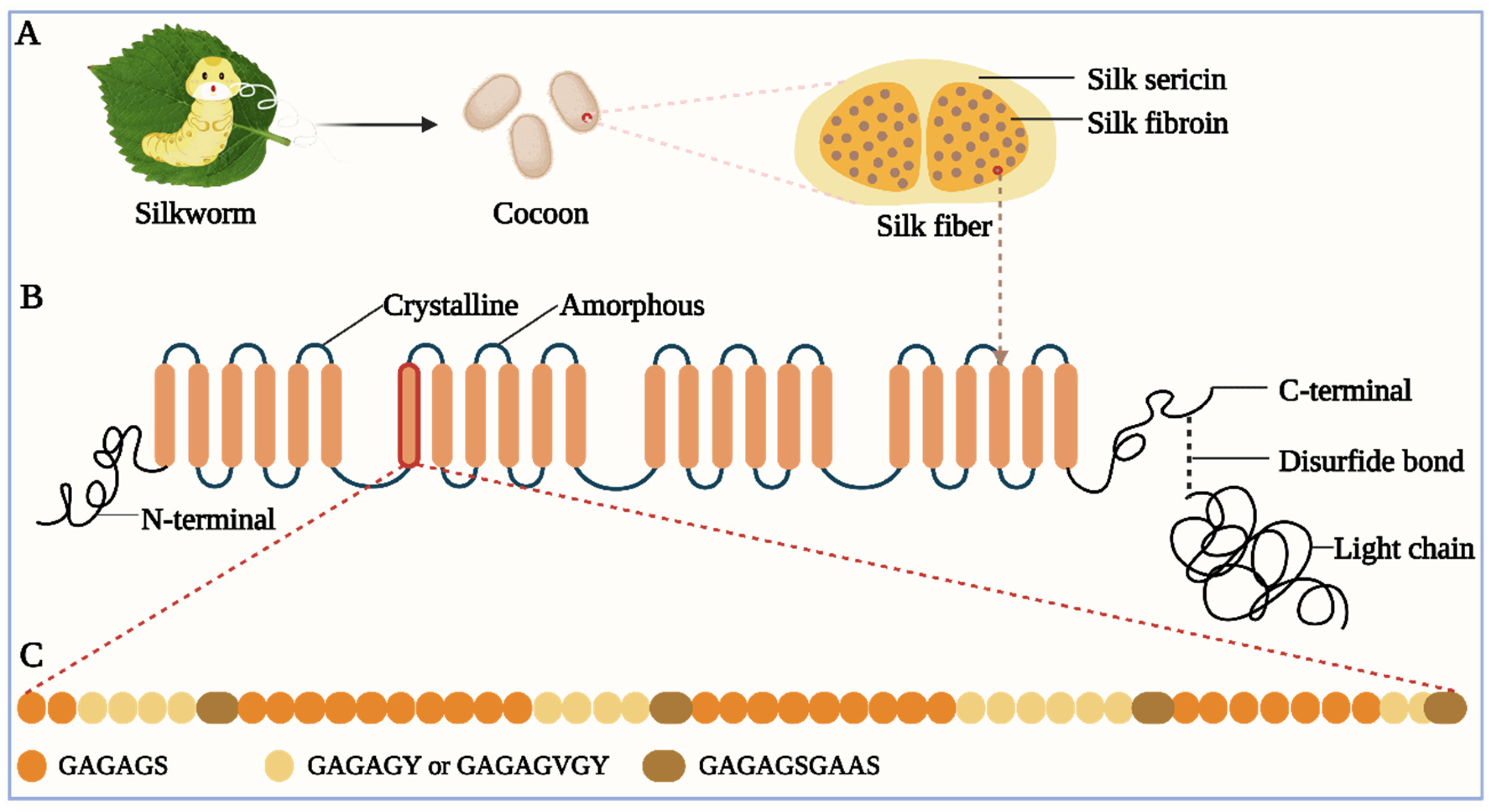
Figure 1. (A) Silk components. The silk cocoon is made of silk fibers spun by silkworms. The silk fiber consists of SF and sericin. (B) Schematic of H-chain. H-chain has four different domains: crystalline domain, amorphous domain, N-terminal, and C-terminal. H-chain and L-chain are connected by a disulfide bond at the H-chain’s C-terminal. (C) Amino acid sequence of the crystalline domain. It includes several repetitive polypeptide segments, mainly including GAGAGS, GAGAGY (or GAGAGVGY), and GAGAGSGAAS.
The SF is a macromolecular and fibrous protein comprising three elementary units: a fibroin heavy chain (H-chain), a fibroin light chain (L-chain), and a glycoprotein (P25) [4][5][6]. The H-chain consists of roughly 5200 amino acid residues, including glycine (45.9%), alanine (30.3%), serine (5.3%), valine (1.8%), and 15 other amino acids. It is composed of four different domains: crystalline domain, amorphous domain, N-terminal, and C-terminal [7][8]. The crystalline domain primarily consists of several repetitive polypeptide segments, of which two hexapeptides (Gly-Ala-Gly-Ala-Gly-Ser (GAGAGS) and Gly-Ala-Gly-Ala-Gly-Tyr (GAGAGY)) account for approximately 70%, simplifying the amino acid sequence of the crystalline domain (Figure 1B,C) [9][10]. The crystalline domain and amorphous domain alternate within the H-chain. The L-chain has no repetitive polypeptide segments and is predominantly composed of alanine and aspartic acid. The H-chain and L-chain are connected by a disulfide bond between Cys-c20 (the cysteine residue located at the 20th position from the C-terminal of the H-chain) and Cys-172 of the L-chain [11]. The bond is crucial for fibroin’s normal secretion from the posterior silk gland. Without it, the secretion of SF will be impeded [12][13]. The dominant crystal structure of natural SF constitutes Silk I and Silk II [14][15]. The Silk II is an antiparallel β-sheet structure and is a stable crystal structure due to the powerful hydrogen bond between neighboring peptide blocks, which imparts excellent mechanical performances such as high tensile strength and toughness to SF [14][15]. Like SF, sericin is also a macromolecular protein. However, it is globular and usually discarded during the silk degumming process [16][17]. It envelopes SF like glue, cementing two SF fibers together into SF, and facilitating the flow of SF in silkworms [18][19]. Silk sericin is generally believed to constitute 18 amino acids, among which the two most abundant amino acids are aspartic acid and serine, which account for approximately 33.4% and 16.7%, respectively [20][21].
The amino acid composition of SF and sericin set the basis for the interaction of silk proteins with metal ions. For one thing, the oxygen atom of carboxyl groups and the nitrogen atom of amino groups in amino acids contain lone pairs of electrons. After dehydration condensation, the oxygen atom and nitrogen atom in amide groups can coordinate with metal ions. In addition, the terminal carboxyl groups, terminal amino groups, and some groups in the side chains of amino acid residues (like the hydroxyl of sericin [22][23]) can also adsorb metal ions through coordination. Furthermore, silk proteins possess amphoteric behavior. When the pH of a solution exceeds its isoelectric point (3.6–5.2 [24][25]), it releases protons, becoming negatively charged, and therefore it can bind with metal ions electrostatically.
2. Interaction of Silk Proteins with Metal Ions
The bond between animal proteins and metal ions deeply affects the function and application of animal proteins. The interaction of silk proteins with metal ions includes electrostatic interaction and chelation [26][27][28][29][30]. The electrostatic interaction is generally the ionic interaction between the negatively charged functional groups of silk proteins and positively charged metal ions. The silk proteins possess abundant polar functional groups with a negative charge, which include -COOH, -NH2, -OH, -CO-NH-, -CO-, and -S-S- [31][32]. These negatively charged functional groups provide binding sites for metal ions. In addition, these polar functional groups contain atoms with lone pairs of electrons, including O and N [33][34][35]. These atoms are prone to chelate with electron-deficient metal ions, forming coordinate bonds. The electrostatic interaction and chelation impart a strong affinity for metal ions to silk proteins. The preferential interactions of an SF peptide and two artificial peptides (RLWRLLWRLWRRLWRLLR (C6M1) and RLLRLLLRLWRRLLRLLR (C6)) with Cu2+ than anthocyanin and the resulting protective effect on anthocyanin set excellent examples [36][37][38].
The formation of silk/metal composites usually results from the combined effect of these two interactions, which can be demonstrated with the following examples. In an ultrafiltration experiment using sericin biopolymer, the removal efficiencies of five metal ions (Pb2+, Co2+, Ni2+, Cu2+, and Zn2+) increased, and their permeate fluxes decreased with increasing sericin dose at neutral pH before reaching a plateau [28]. This was attributed to the higher availability of anionic reactive sites and more atoms containing lone pairs of electrons with increasing sericin dose, leading to more metal ions forming complexes with sericin. The chelation reaction between divalent metal ions and poly dentate ligands (PDLs) of sericin (S), such as NH2-, -S-S-, and -COOH, can be written as Equation (1). Furthermore, this research found that -NH2 was very effective in promoting metal ion binding to sericin and served as an example for illustrating chelation. Stable sericin/metal complexes were formed due to abundant active amino groups chelating with metal ions. The structure of the active amino groups changed with pH: -NH2 tended to be deprotonated at basic pH, making sericin negatively charged, while it tended to be protonated at an acidic pH, making sericin positively charged. In addition, each bivalent metal ion chelated with four -NH2. This illustration of sericin’s charge is consistent with a study in which silk microparticles were used to adsorb Cr6+ in aqueous solutions [39]. The study found that the net charge of sericin was positive at pH 1–2. The reason was that the isoelectric pH of sericin was approximately 4.0 due to carrying more acidic amino acid residues, for example, glutamate acid and aspartic acid, than basic amino acid residues [25][40]. The dominant existing forms of Cr6+ were H2CrO4, HCrO4−, and Cr2O42− at pH below 1, 2–6, and above 6, respectively [41]. At pH 1–2, owing to the electrostatic interaction of the negative metal ions with the positively charged sericin, sericin microparticles adsorbed Cr6+ effectively and the adsorption capacity exhibited a downward trend as pH increased from 1 to 5. Note that the study reflects that although sericin still interacted with metal ions due to a combination of electrostatic interaction and chelation, the charge of the substance participating in the binding and the binding site could be different and varied with reaction parameters, such as pH and the properties of metal ions. Another study also demonstrated that the interaction between sericin and metal ions was dominated by chelation and electrostatic interaction between sericin’s active amino groups and metal ions [42]. Due to the stronger chelation and electrostatic interaction between sericin and metal ions, metal ions in aqueous solutions first interacted with sericin and then with anthocyanin, making anthocyanin a suitable indicator for the saturation state of adsorption for the filter membrane. Due to the formation of coordinate bonds, the adsorption using silk proteins was mainly chemical and follows a pseudo-second-order kinetic model [39][43][44][45].
Metal-Sericin Complexation:
where PDL is short for poly dentate ligand, and S is short for sericin.
Pb2+/Co2+/Ni2+/Cu2+/Zn2+(aq) + n[PDL(S)]2−(aq) → [Pb/Co/Ni/Cu/Zn-PDL(S)n](aq)
3. Factors Influencing the Interaction of Silk Proteins with Metal Ions
Several factors influence the interaction of silk proteins with metal ions, such as pH, contact time [39], temperature [26][43], and metal ion properties [43][46][47][48].
pH plays a crucial role in the chemical formula (partially due to pronation and deprotonation and the surface charges of sericin and metal ions. Therefore, pH is the main influencing factor for the adsorption of metal ions by sericin [39][49]. The adsorption capability is a function of contact time, which can be employed to determine the adsorption kinetic model. Throughout the adsorption process, the adsorption capability of a sericin-based adsorbent typically exhibits a rapid increase, followed by a gradual increase before reaching a plateau. The plateau signifies that the adsorption of metal ions is saturated because active sites are no longer available in the adsorbent materials [39]. The equilibrium time is defined as the duration of the adsorption process, while the maximum or saturated adsorption capacity refers to the amount of adsorbed metal ions. In practical applications, contact time affects the cost-efficiency of adsorbents for metal ions. Temperature is another critical factor influencing the interaction mechanism between metal ions and silk proteins. The adsorption can be classified as either endothermic or exothermic, based on the variation of metal ion adsorption capacity with temperature. If the capacity increases with rising temperature, the adsorption is endothermic, and vice versa. The binding-free energy for silk proteins to different metal ions primarily pertains to the properties of metal ions, including charge-accepting ability, ionic radius, and valence state. The electrostatic interaction between the negatively charged ligands in silk proteins and metal ions, as well as the charge–dipole interaction between metal ions and noncharged ligands in silk proteins, is stronger when metal ions carry a higher net charge. For groups IA and IIA, a larger ionic radius results in a smaller charge density, endowing ions with lower electronegativity and subsequently reducing their affinity to silk proteins. However, this rule does not apply to groups IB and IIB due to significant relativistic effects [50]. For groups IB and IIB, although the ion with a larger radius has a smaller charge density, the relativistic effect leads to a strong stabilization of the vacuum 6s orbital, which enhances the charge-accepting ability and thus its interaction with negatively charged ligands. For instance, Hg2+ and Au+ had a stronger charge-accepting ability than Zn2+ and Ag+ that belong to the same groups in the periodic table, respectively. When two metal ions have similar valence states and radii, the one with a stronger charge-accepting capability demonstrates a greater affinity to silk proteins and forms a more stable silk protein/metal complex than the other, for which the interactions of sericin with Mg2+ and Zn2+ set a good example [50].
This entry is adapted from the peer-reviewed paper 10.3390/su152216053
References
- Dusi, G.G.; Marques, G.S.; Kienteca, M.L.; Gimenes, M.L.; Cerutti, M.L.M.N.; da Silva, V.R. Biosorption investigation of Cu(II) ions from aqueous solutions using sericin–alginate particles: Kinetic, equilibrium, and thermodynamic. Sustain. Chem. Pharm. 2022, 25, 100601.
- Ru, M.; Hai, A.M.; Wang, L.; Yan, S.; Zhang, Q. Recent progress in silk-based biosensors. Int. J. Biol. Macromol. 2023, 224, 422–436.
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K.R. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494.
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528.
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276.
- Rastogi, S.; Kandasubramanian, B. Progressive trends in heavy metal ions and dyes adsorption using silk fibroin composites. Environ. Sci. Pollut. Res. 2020, 27, 210–237.
- Hao, Z.; Long, D.; Zhang, Y.; Umuhoza, D.; Dai, J.; Xu, Z.; Zhang, G.; Meng, W.; Xiang, Z.; Zhao, A. New insight into the mechanism of in vivo fibroin self-assembly and secretion in the silkworm, Bombyx mori. Int. J. Biol. Macromol. 2021, 169, 473–479.
- Fedič, R.; Žurovec, M.; Sehnal, F. Correlation between Fibroin Amino Acid Sequence and Physical Silk Properties. J. Biol. Chem. 2003, 278, 35255–35264.
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470.
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombix mori. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1999, 1432, 92–103.
- Mori, K.; Tanaka, K.; Kikuchi, Y.; Waga, M.; Waga, S.; Mizuno, S. Production of a chimeric fibroin light-chain polypeptide in a fibroin secretion-deficient naked pupa mutant of the silkworm Bombyx mori. J. Mol. Biol. 1995, 251, 217–228.
- Takei, F.; Kikuchi, Y.; Kikuchi, A.; Mizuno, S.; Shimura, K. Further evidence for importance of the subunit combination of silk fibroin in its efficient secretion from the posterior silk gland cells. J. Cell Biol. 1987, 105, 175–180.
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499.
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237.
- Yang, C.; Yao, L.; Zhang, L. Silk sericin-based biomaterials shine in food and pharmaceutical industries. Smart Mater. Med. 2023, 4, 447–459.
- Rong, H.H.; Zhang, M.C.; Liang, X.; Liu, C.; Saadi, M.; Chen, X.Y.; Yao, L.; Zhang, Y.R.; He, N.; Hu, E.R.; et al. Demonstration of electronic synapses using a sericin-based bio-memristor. Appl. Phys. Express 2023, 16, 031007.
- Lee, K.H. Silk sericin retards the crystallization of silk fibroin. Macromol. Rapid Commun. 2004, 25, 1792–1796.
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012.
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100.
- Suryawanshi, R.; Kanoujia, J.; Parashar, P.; Saraf, S.A. Sericin: A Versatile Protein Biopolymer with Therapeutic Significance. Curr. Pharm. Des. 2020, 26, 5414–5429.
- Zhou, K.; Peters, R.J. Investigating the conservation pattern of a putative second terpene synthase divalent metal binding motif in plants. Phytochemistry 2009, 70, 366–369.
- Paul, J.J.; Kircus, S.R.; Sorrell, T.N.; Ropp, P.A.; Thorp, H.H. Effects of coordinating metal ions on the mediated inhibition of trypsin by bis(benzimidazoles) and related compounds. Inorg. Chem. 2006, 45, 5126–5135.
- Qiao, X.Y.; Miller, R.; Schneck, E.; Sun, K. Influence of pH on the surface and foaming properties of aqueous silk fibroin solutions. Soft Matter 2020, 16, 3695–3704.
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064.
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437.
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly Efficient, Irreversible and Selective Ion Exchange Property of Layered Titanate Nanostructures. Adv. Funct. Mater. 2012, 22, 835–841.
- Khosa, M.A.; Shah, S.S.; Feng, X.S. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456.
- Zhong, L.-S.; Hu, J.-S.; Liang, H.-P.; Cao, A.-M.; Song, W.-G.; Wan, L.-J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431.
- Cao, C.-Y.; Qu, J.; Yan, W.-S.; Zhu, J.-F.; Wu, Z.-Y.; Song, W.-G. Low-Cost Synthesis of Flowerlike α-Fe2O3 Nanostructures for Heavy Metal Ion Removal: Adsorption Property and Mechanism. Langmuir 2012, 28, 4573–4579.
- Liu, H.; Sun, Z.; Guo, C. Chemical Modification of Silk Proteins: Current Status and Future Prospects. Adv. Fiber Mater. 2022, 4, 705–719.
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258.
- He, H.-Z.; Zhang, Y.; Li, Y.; Wang, P. Recent innovations of silk-derived electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and oxygen reduction reaction. Int. J. Hydrog. Energy 2021, 46, 7848–7865.
- Bartlett, G.J.; Woolfson, D.N. On the satisfaction of backbone-carbonyl lone pairs of electrons in protein structures. Protein Sci. 2016, 25, 887–897.
- He, H.; Zhang, Y.; Wang, P.; Hu, D. Preparation of sponge-cake-like N-doped porous carbon materials derived from silk fibroin by chemical activation. Microporous Mesoporous Mater. 2021, 317, 110998.
- Li, Y.; Yao, L.; Zhang, L.; Zhang, Y.; Zheng, T.; Liu, L.; Zhang, L. Enhanced physicochemical stabilities of cyanidin-3-O-glucoside via combination with silk fibroin. Food Chem. 2021, 355, 129479.
- Yao, L.; Xu, J.; Zhang, L.; Liu, L.; Zhang, L. Nanoencapsulation of Anthocyanin by an Amphiphilic Peptide for Stability Enhancement. Food Hydrocoll. 2021, 118, 106741.
- Yao, L.; Xu, J.; Zhang, L.; Zheng, T.; Zhang, L. Physicochemical Stability-Increasing Effects of Anthocyanin via a Co-Assembly Approach with an Amphiphilic Peptide. Food Chem. 2021, 362, 130101.
- Kwak, H.W.; Kim, Y.; Yun, N.K.; Lee, K.H. Silk sericin microparticles as a biosorbent for hexavalent chromium ion. Macromol. Res. 2014, 22, 788–795.
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Chapter Six—Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 98, pp. 169–221.
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12.
- Yao, L.; Hao, M.; Zhao, F.; Wang, Y.; Zhou, Y.; Liu, Z.; An, X.; Gao, Z.; Wang, J.; Zheng, T.; et al. Fabrication of silk sericin-anthocyanin nanocoating for chelating and saturation-visualization detection of metal ions. Nanoscale 2022, 14, 17277–17289.
- Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions. Int. J. Mol. Sci. 2016, 17, 1466.
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52.
- Zhang, K. The Extraction of Silk Fibroin & Preparation, Modification and Application of Silk Fibroin Membrane; Dong Hua University: Shanghai, China, 2015.
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479.
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152.
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2014, 114, 538–556.
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013, 3, 20800–20810.
- Tai, H.-C.; Lim, C. Computational Studies of the Coordination Stereochemistry, Bonding, and Metal Selectivity of Mercury. J. Phys. Chem. A 2006, 110, 452–462.
This entry is offline, you can click here to edit this entry!
