Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
There are multiple benefits offered by capsule endoscopy (CE). First, the patients do not need sedation to undergo a CE analysis. The CE can analyze the entire GI tract from the esophagus, passing through the stomach, until the small intestine, which could not be properly analyzed through conventional endoscopy. The capsule has the size of a conventional vitamin capsule, and it can be easily swallowed, moving naturally through the GI tract until excretion. This fact indicates a painless procedure compared with the discomfort suffered by the long endoscopy sessions.
- capsule endoscopy
- endomicroscopy
- endoscopy
- GI tract
1. Indications of Esophageal Capsules
1.1. Screening for Barrett’s Esophagus
Barrett’s esophagus consists of a metaplastic change of the esophageal mucosa’s lining, meaning that the columnar epithelium replaces the squamous epithelium that normally overlays the distal esophagus [45,46,47]. Barrett’s esophagus is an important risk factor for indication of esophageal adenocarcinoma, and several studies have indicated that its incidence has increased rapidly over the years [48,49]. The studies comparing the diagnostic yield between CE and conventional EGD demonstrated that CE was feasible, safe, and well tolerated by the patients. Moreover, the patients always preferred CE over unsedated EGD. On the other hand, the sensitivity of the esophageal capsule was variable between the detection of BE and another esophageal disease: 60–100% and 50–89%, respectively [50,51]. Although the results in terms of sensitivity are promising, studies have suggested that EGD is more cost-effective than CE for BE screening [52].
1.2. Screening for Esophageal Varices
The use of CE for detecting esophageal varices is not well defined due to the fact that all the studies present considerable heterogeneity between their findings.
Pena et al. found that an esophageal capsule could be used in the assessment of esophageal varices (EV). The sensitivity calculated in this study was 68.4% in detecting EV using CE against 95% using EGD. However, due to the minimal discomfort, lack of sedation, and low risk offered by CE, this technology is a possible substitute for EGD [53].
Groce’s study showed a sensitivity of CE in detecting EV around 78% and that CE may be superior to EGD for identification of small EV [54]. On the other hand, Einsen’s and Smith’s study indicated a better perspective on CE tests, with sensitivity reaching up to 100% [55,56]. The same results were found in Ragunath’s study [57]. The lowest sensitivity was showed in Jensen’s study, where it was only 8.3%, with modest accuracy of the CE in the identification of EV [58].
2. Indications of Intestinal Capsules
2.1. Intestinal Tumors
Tumors found in the small bowel (SB) represent 5% of all GI tract tumors and 2% of the cancer rates, despite a very low accuracy of the estimative, since the current methodologies have been proven to be inadequate [59]. On the other hand, the investigation of small bowel tumors with CE, an effective diagnostic modality, was established in 2004, and 8.9% of the patients who underwent the procedure were diagnosed with SB tumors. The expectation of the clinicians was that CE may lead to earlier detection and treatment of SB tumors, thereby improving the results for patients with neoplasms [60].
2.2. Obscure GI Bleeding
Obscure GI bleeding can be defined by episodes of digestive bleeding, a positive fecal occult blood test, or chronic iron-deficiency anemia [61,62]. The complexity of GI bleeding relates to the fact that the bleeding can occur from multiple lesions at many sites in the GI tract. This pathology is evident to the patient but can be from a source which is not easily identifiable via conventional upper or lower endoscopy [63]. For that reason, and based on meta-analysis studies, the diagnostic yield of intestinal capsules ranges from 55% to 81%, which confirmed the superiority of CE diagnosis against other modalities of conventional endoscopy [64,65,66]. Figure 2 shows the small bowel findings of obscure GI bleeding achieved with intestinal CE.
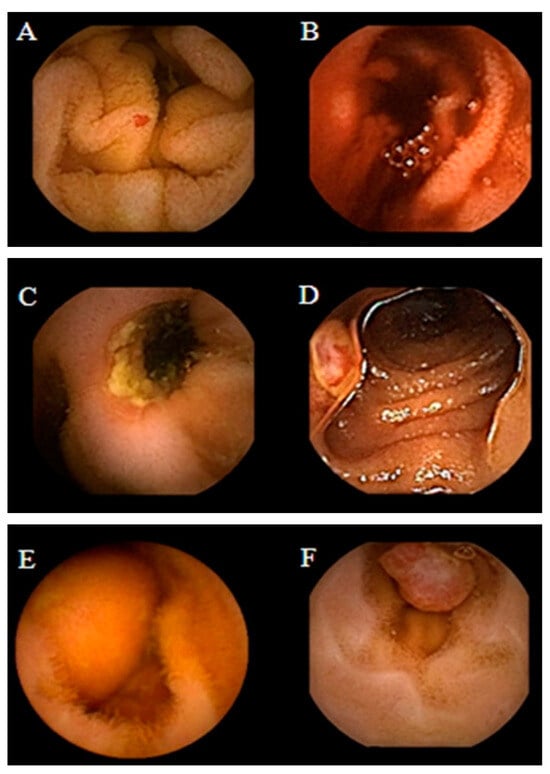
Figure 2. Spectrum of small bowel findings identified via capsule endoscopy (CE) in patients with obscure gastrointestinal bleeding (OGIB). (A) Nonbleeding angioectasia, (B) active bleeding, (C) ulcer with stenosis, (D) small bowel erosion, (E) submucosal mass, and (F) polyp [67].
2.3. Crohn’s Disease
Crohn’s disease is an inflammatory disease of the GI tract and often spreads deep into the layers of affected tissue. It can occur from the mouth to anus [68], although the probability of its incidence in the small bowel ranges from 30% to 40% of cases [69,70].
Generally, in order to identify an occurrence of Crohn’s disease, the clinicians must rely on a combination of clinical, endoscopic, and histological findings, because there is no single test that can fully diagnose the disease. Imaging studies normally lack sensitivity to be able to identify early lesions [29].
Schulmann et al. tested capsule endoscopy for the purpose of finding evidence of Crohn’s disease, and at that time, they agreed that full visualization and imaging of the entire length of the SB was unsatisfactory, and CE was considered a promising new approach for the diagnosis of SB diseases [71].
Albert et al. compared CE with magnetic resonance imaging (MRI) and found that CE can detect limited mucosal lesions that may be missed by MRI and was slightly more sensitive than MRI: 12 versus 10 of 13 in suspected Crohn’s disease and 13 versus 11 of 14 in established Crohn’s disease [72]. Based on the stated facts, CE proved to be a good complementary method for diagnosing SB Crohn’s disease. More recently, Sange et al. state that CE innovation reduced reading time, improved diagnostic accuracy, and enhanced image quality [73]. Figure 3 presents findings of active Chron’s disease detected via intestinal CE.
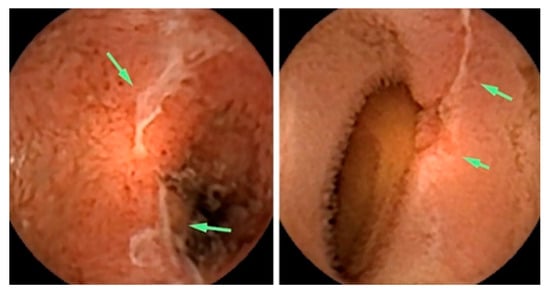
Figure 3. Active Crohn’s disease in the more proximal small bowel (SB), detected via CE. Images shown are ulcers detected via small bowel CE in the proximal SB. The green arrows indicate the ulcers [74].
2.4. Celiac Disease
The use of capsule endoscopy in patients with celiac disease consists of finding complications such as unexplained diarrhea, abdominal pain, and small bowel tumors. Fry et al. found a low yield for capsule endoscopy in patients with abdominal pain or diarrhea, and they recommended this type of evaluation as a first-line test. Moreover, the results showed a yield of 6% for abdominal pain, 14% for diarrhea, and 13% for both [75]. Based on the experiments conducted by [76,77,78,79] and presented at the ICCE consensus, for the diagnosis of Celiac disease with CE, Cellier et al. considered that there was enough evidence to support the use of CE in patients who have been treated and previously confirmed to have celiac disease [80].
2.5. Genetic Disorders
Soares et al. performed a study about Peutz-Jeghers syndrome (PJs)—an inherited gastrointestinal polyposis disorder, most commonly found in the small intestine. They found that CE offered excellent visualization of the small intestine and correctly identified all the patients having large polyps, although it missed 20% of the total number of them [81].
3. Commercial Capsules
Since the early years of conventional endoscopic procedures, the small bowel has been considered technically difficult to examine due to its length and location. The concept of a capsule indicated for small bowel analysis was developed by two groups. In 1996, a gastroenterologist named Dr. Paul Swain demonstrated the first live transmissions of a capsule analysis using a pig’s stomach. In 1997, he decided to collaborate with Dr. Gavriell Iddan, a mechanical engineer [14,82,83]. In 2000, they published successful animal trials [82], and in 2001, they published human studies on the use of capsule endoscopy in clinical trials [14,84]. At this time, the Food and Drug Administration (FDA) approved the capsule endoscopy [62].
Previously, the small bowel was a difficult organ to explore with the available technologies, such as conventional endoscopy or radiological and nuclear techniques, due to anatomical or physiological causes. In 2005, the role of the capsule endoscopy was widely discussed at the International Conference on Capsule Endoscopy (ICCE) [81,85,86,87,88,89,90], a symposium organized and sponsored by Given Imaging.
Nowadays, there are several brands of capsules which are approved by the FDA, like PillCam by Given Imaging, OMOM by Jinshan Science & Technology, EndoCapsule by Olympus, and MiroCam by Intromedic (Seoul, Republic of Korea). Next, we describe each capsule, its advantages, drawbacks, and main components.
The approval from the FDA for the PillCam SB capsule was received in 2003, and the market release was indicated for use in pediatric patients, aiming specifically at the diagnosis of pathologies of the small bowel [65,91].
The PillCam capsules produced by Given Imaging Inc., Duluth, GA, USA, are divided into three categories, small bowel (SB), esophagus (ESO), and colon (COLON), which have video cameras designed for imaging the gastrointestinal tract. Each of them is equipped with a battery, LEDs (light-emitting diodes), and a transmitter with an antenna. All these components are enwrapped in a biocompatible plastic casing, and the capsule size is about 26.4 mm length and 11.4 mm diameter [92].
The PillCam SB category is subdivided into SB, SB2, and SB3. PillCam SB capsules incorporate one video camera. PillCam SB2 consists of a fixed-frame-rate second-generation capsule, and the PillCam SB3 has enhanced imaging capabilities with an adaptive frame rate (AFR) [92]. These capsules are indicated for the monitoring of lesions that may show Crohn’s disease that is not detected through upper and lower endoscopy. They are not indicated for patients with GI obstruction, strictures, or fistulas, patients with cardiac pacemakers or any implanted electro-medical devices, and for patients with dysphagia or other swallowing disorders.
The PillCam ESO category has two variants—PillCam ESO 2 with a fixed high-frame rate and PillCam ESO 3 with a fixed high-frame rate and enhanced imaging capabilities [92]. The PillCam ESO capsules are composed of two video cameras, and they can be used for the investigation of esophageal disorders, such as esophageal varices, esophagitis, and Barret’s esophagus [93], and in patients complaining about heartburn [94,95].
The PillCam COLON capsules also contain two video cameras, and this category is divided into two variants—COLON 1 has a fixed-frame-rate capsule, while COLON 2 has an enhanced imaging capability, AFR- [92]. Both PillCam ESO and PillCam COLON are contraindicated for patients with the profile already described in the PillCam SB section. This capsule is indicated to investigate intestinal disorders and tumors.
After various studies showing the risks of capsule retention, Given Imaging Inc. created the Patency Capsule (PC) System, an ingestible, dissolvable, and disposable capsule, composed of biocompatible materials. The patency capsule is given before a video capsule endoscopy in order to prevent or minimize the risk of capsule retention [96]. The body of the capsule is composed of compressed lactose that dissolves in GI liquids and 5% barium sulfate, which makes the capsule radiopaque. The system is composed of a nonvideo disintegrating capsule, radiofrequency identification (RFID) tag, and an RFID scanner [97]. The PC is supposed to remain intact in the GI tract for about 80 h according to its design, and after that, if not excreted yet, it disintegrates spontaenously [98].
In 2005 Jinshan Science and Technology Company (Chongqing, China) released to the market the OMOM CE. This CE is indicated for investigating obscure gastrointestinal bleeding (OGIB), abdominal pain or diarrhea, partial intestinal obstruction, suspected inflammatory bowel disease, and tumors [99]. A study showed that the visualization of the entire small bowel was achieved in 75% of patients who had undergone the procedure with the OMOM. In the patients with suspected small bowel (SB) disease, the detection of abnormalities was 70.5%. The diagnostic yield for patients with OGIB was 85.7%, while the detection in cases of abdominal pain or diarrhea was around 53.3% [99].
Olympus Medical Systems (Olympus, Tokyo, Japan) received marketing clearance from the FDA in 2007 for their EndoCapsule endoscope system [100]. This capsule was compared with the PillCam SB and classified with the same quality level by Pennazio [28] et al. The EndoCapsule capsule contains a camera, a transmitter, batteries, and a light source. It differs from the PillCam capsule in that it has a high-resolution image chip and an external real-time viewer [101,102].
A prospective randomized comparison between both capsules—Given PillCam SB and Olympus EndoCapsule—was carried out by Hartmann et al. In this study, it was shown that the Olympus EndoCapsule could detect more GI bleeding sources than the Given PillCam SB, although the difference was only numerical and statistically nonsignificant [103].
The Korean MiroCam (Intromedic) is another capsule with similar components to the capsules from Given Imaging and Olympus. The first clinical trial using MiroCam was in 2009, involving 45 patients. The quality of the image was rated as good in 91.1% of the cases, and the transmission rates of the captured image in the stomach, small bowel, and colon were 99.5%, 99.6%, and 97.2%, respectively. The authors disclosed that MiRo was safe and effective for investigating the entire SB, offering a good image quality and real-time feasibility [101].
Moreover, a study carried out in 2012 showed that the evaluation of the entire SB using MiRoCam was achieved in 96% cases, and relevant lesion findings occurred in 58% of patients. They also considered MiRoCam a safe and effective tool for exploring SB with a high completion rate [104]. Table 1 summarizes the commercial capsules available, as well as the indications, imaging system, size, and respective references with studies about each type of capsule.
Table 1. Commercial capsules.
| Capsule | Company | Indication | Imaging System | Size (Diam. × Length) | References |
|---|---|---|---|---|---|
| PillCam SB | Given Imaging Inc. | For pediatric patients; diagnosis of small bowel pathologies | 1 video camera; 2 fps | 11.4 mm × 26.4 mm | [65,91] |
| PillCam SB2 | Given Imaging Inc. | For diagnosis of small bowel pathologies | 1 video camera with fixed high frame rate; 2 or 4 fps | 11.4 mm × 26.3 mm | [92] |
| PillCam SB3 | Given Imaging Inc. | For diagnosis of small bowel pathologies | 1 video camera; enhanced imaging capabilities and AFR; 2 fps or 2–6 fps | 11.4 mm × 26.2 mm | [92] |
| PillCam ESO 2 | Given Imaging Inc. | For investigation of esophageal disorders | 2 video cameras; 19 fps | 11.4 mm × 26.4 mm | [92,93,94,95] |
| PillCam ESO 3 | Given Imaging Inc. | For investigation of esophageal disorders | 2 video cameras; 35 fps | 11.6 mm × 31.5 mm | [92,93,94,95] |
| PillCam COLON | Given Imaging Inc. | For investigation of intestinal disorders | 2 video cameras and AFR; 4 fps | 11.4 mm × 31 mm | [92] |
| PillCam COLON 2 | Given Imaging Inc. | For investigation of intestinal disorders | 2 video cameras and AFR; 4–35 fps | 11.6 mm × 31.5 mm | [92] |
| Patency System | Given Imaging Inc. | For detecting obstructions or strictures in the GI tract | None (radiofrequency identification (RFID) detected by an RFID scanner) | 11.4 mm × 26.4 mm | [97] |
| OMOM | Jinshan Science and Technology Company | For investigation of OGIB, abdominal pain or diarrhea, inflammatory bowel disease, and tumors | 1 video camera; 2 fps | 13 mm × 27.9 mm | [99] |
| EndoCapsule | Olympus Medical Systems | Detection of GI bleeding | 1 video camera and high-resolution image chip | 11 mm × 26 mm | [28,100,101,102] |
| MiRoCam | Intromedic | For investigation of stomach, small bowel, and colon | 1 video camera; 2 fps | 10.8 mm × 24 mm | [102,104] |
4. New Functionalities for Capsule Endoscopy
Although numerous results from clinical studies and experiments of endoscopic capsules are being presented, the unfeasibility of motion control of the capsule makes the diagnostic of the gastrointestinal tract insufficiently accurate. Since the impossibility of any motion control of the capsule has arisen, studies about possible solutions for motion control have been described in some patents [105,106,107,108].
The basic concept behind these patents were capsules being intrinsically controllable by including induction coils or magnetic parts inside an invented capsule structure in the interest of making it responsive to an external magnetic field. However, as this kind of solution required a characteristic design, like the structure of capsule, geometrical shape, and magnetic properties, the costs of these would be raised.
Moreover, a different solution by Carpi et al. [109] was disclosed in 2006, which on the contrary allowed for the control of a traditional and commercially available endoscopic capsule without any structural modification. Their solution proposes a technique that exploits magnetic shells to be applied on traditional capsules prior to their use [109].
Figure 4 illustrates the personal vision of the President of Olympus, Mr. Shimoyana, during his talk at the MicroMachine Summit 2005 [110]. From his view, the capsule endoscopy would have a rapid growth compared with the conventional endoscopy. The reason for this increase might be because of all the functionalities that can be integrated into the endoscopic capsule. The growth rate is directly related to the number of possible functions that can be incorporated in the endoscopic capsule.
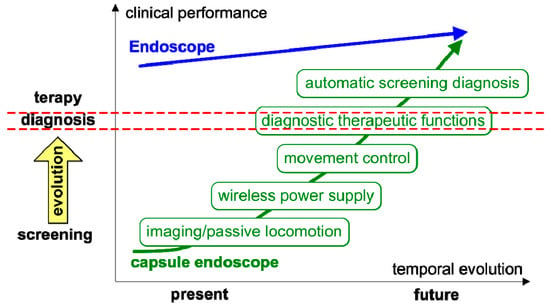
Figure 4. Roadmap for capsule endoscopy presented by Mr. Shimoyana, the President of Olympus, during the MicroMachine Summit 2005 (1–4 May 2005, Richardson, TX, USA) [110]. The red dotted mark the separation of diagnostic functions from therapy functions and simple visualization.
In this context, it is important to discuss the new technologies that can be integrated into the capsule endoscopy in order to increase the performance in screening, diagnosis, and therapy. In the following sections, we present the concept of confocal laser endomicroscopy, photodynamic therapy, narrow-band imaging, and how those technologies can be integrated into the endoscopic capsules.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10121347
This entry is offline, you can click here to edit this entry!
