Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Biomimetic delivery systems (BDSs), defined by their ability to mimic biological systems, hold significant promise in the realm of biomedicine and nanomedicine. They leverage the principles of nature, emulating the structural or functional attributes of biological systems to enhance drug delivery capabilities.
- biomimetic
- bioinspired
- nanodiscs
- liposomes
- virus-like particles
1. Introduction
Biomimetic delivery systems (BDSs), defined by their ability to mimic biological systems, hold significant promise in the realm of biomedicine and nanomedicine. They leverage the principles of nature, emulating the structural or functional attributes of biological systems to enhance drug delivery capabilities [1,2,3]. BDSs often involve the use of naturally derived materials (Figure 1), the structural mimicry of biological entities, or the replication of biological processes, with the aim of improving drug delivery outcomes such as targeting ability, controlled release, and biocompatibility [4,5,6]. Recent advancements in biomimicry have resulted in the creation of innovative drug delivery systems [7,8,9] spanning various paradigms, such as liposomal carriers [10], virus-like nanoparticles (VLPs) for gene delivery [11,12,13], and hydrogel structures [14,15,16]. Additionally, new classes of delivery vehicles have emerged, including extracellular vesicles (EVs) [17,18], red blood cell (RBC)-based carriers [19,20], and nanodiscs (NDs), each presenting unique therapeutic prospects. EVs, naturally occurring cellular delivery systems, comprised of microvesicles and exosomes [21,22], hold promise due to their bio-compatibility and targeted delivery capability [23,24], stimulating interest in their use for delivering RNA-based therapeutics [21,25]. RBCs, with their advantageous properties such as a long circulatory half-life and immune evasion, are under investigation as potential drug carriers, with methods involving their engineering and manipulation into biomimetic nanoparticles [26,27,28]. NDs, mimicking high-density lipoproteins (HDL) [29,30], are versatile delivery platforms due to their ability to solubilize and present various drug molecules; additionally, they have potential benefits for targeted cancer therapy due to their preferential uptake by cancer cells [31,32].
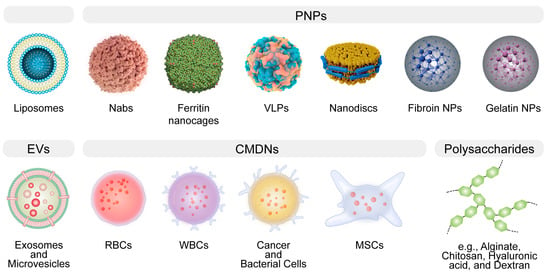
Figure 1. The general illustration of biomimetic delivery systems (BDSs). BDSs are designed to emulate natural structures, thereby augmenting therapeutic efficacy. Notable examples encompass liposomes, protein-based nanoparticles (PNPs), extracellular vesicles (EVs), cell membrane-derived nanocarriers (CMDNs), nanodiscs, and polysaccharides.
The theoretical bedrock of biomimetic delivery systems (BDSs) is fundamentally rooted in the principles of self-assembly, molecular recognition, and biocompatibility [1,2,3]. Self-assembly refers to the process by which molecules spontaneously organize into ordered structures [33,34]. This characteristic, borrowed from nature, is widely harnessed to construct nanoscale delivery vehicles [35]. Molecular recognition refers to the ability of molecules to interact specifically with others, typically resulting in a biological function or response. This principle allows for the precise targeting of therapeutic agents to disease sites, minimizing off-target effects. Lastly, the nano-bio interface effect and biocompatibility are critical attributes of any biomimetic nanosystem intended for clinical use, ensuring that the system does not elicit adverse immune responses or toxic effects [36,37]. The paradigm of drug delivery has seen revolutionary advancement with the burgeoning interest in BDSs, which intimately mimic biological structures to enhance therapeutic efficacy [38,39,40].
These advancements have catalyzed previously unattainable therapeutic opportunities, including targeted cancer therapies [41], gene editing [42], and regenerative medicine [43]. The diversity and adaptability of these BDSs underscore the significant potential of leveraging nature’s design in the development of next-generation therapeutic interventions. However, the path from the bench to bedside translation is fraught with complexity. Despite the theoretical advantages of BDSs, their translation into clinical applications has been slower than expected, hindered by various technical, biological, and regulatory challenges. For instance, issues such as scalability of production, immunogenicity, stability of the systems under physiological conditions, and navigating regulatory approvals pose significant hurdles. The urgency for such a discourse is evident. The promise of biomimicry in healthcare can only be realized when these delivery systems transition from being experimental novelties to tools readily available in the clinician’s arsenal.
2. An Overview of the Strengths and Weaknesses of BDSs
In the rapidly evolving landscape of drug delivery, BDSs stand out as a beacon of innovation, drawing inspiration from biological structures and processes to optimize therapeutic delivery. By mimicking nature, BDSs aim to overcome the myriad challenges associated with traditional drug delivery, ranging from off-target effects to limited bioavailability [5]. BDSs span a broad spectrum, from liposomal structures to protein-based nanoparticles and CMDNs [1,8,42,44]. While the promise of BDSs is undeniable, it’s imperative to evaluate their strengths and weaknesses in comparison with each other (Table 1).
Table 1. An overview of strengths, weaknesses, and therapeutic applications of BDSs.
| BDS | Strengths | Weaknesses | Therapeutic Applications |
|---|---|---|---|
| Liposomes | Biocompatible, versatile in drug loading | Limited stability, potential for rapid clearance | Anticancer and antifungal therapy |
| Protein-based NPs | |||
| Albumin NPs | Natural origin, good safety profile | Variable drug loading efficiency | Anticancer drug delivery |
| Protein-based nanocages | Defined structure, biodegradable | Complex production | Enzyme replacement therapy, vaccine delivery |
| VLPs | High immunogenicity, targeted delivery | Production challenges | Vaccines, cancer immunotherapy |
| NDs | Membrane protein stabilization, defined size | Limited drug loading | Drug and vaccines delivery, drug discovery |
| Silk Fibroin | Biocompatible, high mechanical strength, thermal stability | Potential immunogenicity, variable degradation rates, processing challenges | Bone tissue engineering, wound healing, anticancer drug delivery |
| Gelatin | Biodegradability, ease of modification | Potential risk of disease transmission, temperature sensitivity. | Drug delivery, tissue engineering |
| EVs | Natural origin, low immunogenicity | Isolation purity challenges | Regenerative medicine, anticancer therapy |
| CMDNs | Mimics natural cells, targeted delivery | Complex production | Targeted drug delivery, immunotherapy |
| Polysaccharides | |||
| Alginate | Biocompatible, gel-forming | Rapid degradation in vivo | Wound healing, drug delivery |
| Chitosan | Biocompatible, mucoadhesive | Limited solubility in neutral and alkaline pH | Wound healing, vaccine delivery |
| Hyaluronic acid | Biocompatible, natural targeting to CD44 receptors | Rapid degradation in vivo | Osteoarthritis treatment, drug delivery |
| Dextran | Soluble, biocompatible | Potential for hypersensitivity reactions | Iron-deficiency treatment, drug delivery |
Liposomes are spherical vesicles composed of phospholipid bilayers that can encapsulate a wide variety of therapeutic agents. Their biocompatibility arises from their resemblance to biological membranes, making them a preferred choice for drug delivery [45]. Despite their adaptability in drug loading, liposomes are not without limitations [46]. A critical issue pertains to their stability, which can be compromised during storage, necessitating the development of sophisticated stabilization strategies to ensure the longevity and efficacy of the liposomal formulation [47,48,49,50]. In vivo, liposomes may exhibit rapid clearance from the bloodstream, primarily due to opsonization and subsequent phagocytosis by the cells of the mononuclear phagocyte system [51,52]. This necessitates careful consideration of liposome size, surface charge, and surface modification with polymers such as polyethylene glycol (PEG) to extend their circulatory half-life [51,53].
Protein-based NPs, encompassing albumin NPs, protein-based nanocages, VLPs, and NDs, offer a versatile toolkit for enhancing drug delivery, each with distinct advantages and shared challenges. Albumin NPs utilize human serum albumin, which has a natural propensity to bind to various substances, thereby facilitating the transport of a wide range of molecules [54]. The biodegradability and lack of immunogenicity of albumin contribute to its appeal as a drug carrier. Notably, albumin has a unique ability to accumulate in tumor tissues due to the enhanced permeability and retention (EPR) effect, making it particularly useful for oncological applications [55,56]. However, the drug loading efficiency of albumin NPs can be unpredictable, and their interaction with the biological environment may sometimes lead to rapid clearance from the circulatory system. Despite this, the clinical success of albumin NPs is exemplified by the FDA-approved drug Abraxane, which is an albumin-bound form of paclitaxel used for the treatment of various cancers [57]. Protein-based nanocages are a novel form of protein NPs that offer a highly structured and uniform platform for drug delivery [58]. They are engineered by utilizing the self-assembling properties of certain proteins to form cage-like structures that can encapsulate therapeutic agents within their hollow interior [59]. This allows for precise control over the dosage and protection of the cargo from enzymatic degradation. However, the complexity of synthesizing these nanocages poses a significant challenge, potentially limiting their rapid deployment in clinical settings [60]. VLPs are multiprotein structures that mimic the organization and conformation of viruses but are devoid of viral genetic material, which mitigates safety concerns associated with live viral vectors. The repetitive, high-density display of antigens on their surface makes VLPs particularly effective as vaccine platforms, eliciting strong immune responses [11,61,62]. However, the production of VLPs is technically demanding, often requiring cell culture systems, and the scale-up for mass production can be challenging [63,64,65]. Nanodiscs are synthetic nanoscale particles that incorporate membrane proteins within a phospholipid bilayer stabilized by scaffold proteins. NDs provide a unique milieu for the study of membrane proteins in their near-native state, which is invaluable for drug discovery and development [66]. While they offer a controlled environment for membrane proteins, their therapeutic application as drug delivery vehicles is still nascent [30,67,68], with issues related to production scalability and drug loading capacity yet to be fully addressed. In a comparative context, while albumin NPs have achieved clinical use, protein-based nanocages and VLPs are still primarily in the research or early clinical trial stages. NDs, being relatively recent developments, and have not yet been extensively explored for therapeutic delivery but hold potential due to their unique ability to present membrane proteins and delivery of lipophilic drugs. Each of these protein-based NPs has its advantages in terms of specificity, biocompatibility, and targeting ability; however, they also face common challenges such as production complexity, stability, and potential immunogenicity.
Silk fibroin (SF) and gelatin (GA) epitomize the contrasting paradigms within BDSs, each with inherent strengths and challenges. SF is distinguished by its robust mechanical properties and sustained release potential, making it a quintessential candidate for structurally demanding applications such as in bone tissue engineering and targeted cancer therapies [69,70,71]. Nevertheless, its utility is occasionally circumscribed by intricate processing requirements and immunogenic concerns. Conversely, GA is celebrated for its facile chemical modifiability and hydrogel formation aptitude, characteristics that are pivotal for localized therapeutic delivery and tissue engineering scaffolds [72,73]. Yet, its application is sometimes compromised by inferior mechanical integrity, thermal instability, and the latent risk of pathogenic transmission [74]. The selection between SF and GA for DDSs is thus dictated by a nuanced balance between the therapeutic context and the material’s physicochemical congruity, with each material offering distinctive contributions to the diversifying landscape of biomimetic therapeutic delivery.
EVs and CMDNs represent two innovative approaches in the realm of biomimetic drug delivery, each leveraging the innate properties of cellular components. EVs, owing to their natural origin, can transport a wide variety of biomolecules and have the ability to cross biological barriers with a low risk of immune response, positioning them as promising vectors for regenerative medicine and targeted cancer therapies [18,75,76]. Nevertheless, isolating EVs with high purity remains a significant technical challenge [77,78,79]. CMDNs, on the other hand, utilize the unique attributes of cell membranes to cloak nanoparticles, enabling them to evade the immune system and increase delivery specificity [27,80,81]. This strategy has shown considerable promise in targeted drug delivery and immunotherapy, capitalizing on the natural homing abilities of cells. Both EVs and CMDNs still face substantial production complexities (EVs in terms of isolation and CMDNs with membrane extraction and nanoparticle integration).
Polysaccharides, a diverse group of biopolymers, including alginate, chitosan, hyaluronic acid, and dextran, play a pivotal role in the landscape of therapeutic delivery due to their inherent biocompatibility and tailored biodegradability [82,83]. Alginate, renowned for its gel-forming capabilities, is widely used in wound healing applications and as a matrix for cell encapsulation, benefiting from its gentle gelation conditions that preserve cell viability [84,85]. Chitosan, with its distinctive mucoadhesive properties and ability to open tight junctions [86], is exploited for enhanced mucosal delivery of drugs, offering improved bioavailability and prolonged retention at the site of administration. Hyaluronic acid, by virtue of its specific interaction with CD44 receptors [87], which are overexpressed in many cancer cells, has emerged as a targeted delivery vehicle, especially in the treatment of osteoarthritis, where it can provide both viscosupplementation and targeted relief [88]. Dextran, due to its excellent solubility and minimal toxicity, is employed in various drug delivery systems and as a plasma volume expander, with its iron-conjugated forms used to treat iron-deficiency anemia [89,90].
Despite these advantages, the application of polysaccharides is not devoid of challenges; their susceptibility to rapid degradation in vivo may limit their utility, and potential immunogenicity cannot be entirely discounted. Moreover, the batch-to-batch variability and the complexity of producing highly purified, well-characterized polysaccharides can impact the reproducibility and scalability of pharmaceutical products. Hence, while polysaccharides offer considerable benefits for drug delivery, their clinical application requires meticulous optimization to ensure efficacy, safety, and manufacturability.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15112623
This entry is offline, you can click here to edit this entry!
