Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Endometriosis is a chronic condition characterized by the presence of abnormal endometrial tissue outside the uterus. These misplaced cells are responsible for inflammation, symptoms, scar tissue and adhesions. Endometriosis manifests mainly in three patterns: superficial peritoneal lesions (SUP), ovarian endometriomas (OMA) and deep infiltrating endometriosis (DIE). It also exhibits atypical and extremely rare localization. The updated 2022 guidelines of the ESHRE recommend using both ultrasound and magnetic resonance imaging (MRI) as first-line diagnostic tests.
- endometriosis
- MRI
- deep pelvic endometriosis
- endometrioma
- adenomyosis
- post-operative imaging
1. Introduction
Endometriosis is defined as a benign chronic inflammation caused by the abnormal presence of endometrial glands and stroma outside the uterus. The ectopic endometrial cells on the surface of other organs, such as the ovaries (endometrioma), uterus (adenomyosis), tube, intestine, vagina, and bladder, undergo the same modifications of the physiological uterine endometrium during the menstrual cycle; this can generate an inflammatory state with symptoms and the formation of scar tissue and adhesions that, if neglected, can also cause infertility [1].
Several theories exist concerning the origins of endometriosis, with the most widely accepted one suggesting that endometrial cells shed during menstruation make their way through the fallopian tubes into the abdominal cavity. This leads to their implantation on the peritoneum and the surface of the pelvic organs [2]. However, this hypothesis does not rule out alternative explanations, including a potential genetic factor, the spread of endometrial cells through the bloodstream and lymphatic system, or the metaplastic theory. Specifically, the latter theory proposes that both endometrial and peritoneal cells originate from a shared embryological precursor [3]. Therefore, it suggests that peritoneal cells have the potential to undergo a transformation into endometrial cells for reasons that are currently unknown [4]. The peak incidence occurs between the ages of 25 and 35, but the disease can also occur in lower age groups. Diagnosis of the condition is not always immediate and may often involve a prolonged diagnostic journey [5].
Endometriosis can be asymptomatic. When symptomatic, it usually manifests with pelvic pain, especially in the peri-menstrual phase, painful menstruation (dysmenorrhea), pain during sexual intercourse (dyspareunia), urinary urgency, hematuria or pain during defecation, sometimes together with the presence of blood in the faeces [1].
2. Indications for MRI
MRI is able to recognize past hemorrhagic content in endometrioma, and owing to its large field of view (FOV), spatial resolution and contrast imaging, can identify multiple dislocated endometrial implants [14]. It is, therefore, often requested as a second-line technique in the case of still symptomatic patients without any ultrasound findings [15], for patients undergoing surgery for pre-operative staging and mapping of the implants, or in case of difficult diagnosis [16]. Only a few recent studies suggest the use of MRI as a triage test in the diagnosis of rectosigmoid colon endometriosis and as a first-line examination in women with a high suspicion of intestinal endometriosis [17].
2.1. Procedure and Patient Preparation
There is no consensus regarding patient preparation [11]: fasting is the most suggested for evaluation of DIE imaging for at least three to six hours before the exam. It is also recommended to refrain from voiding for 1 h before the MRI, so as to reach a correct angle of the uterus and displace the small bowel [14], and to improve the detection of small implants in the anterior compartment. An extremely filled bladder could in fact cause spastic movements of the detrusor muscle with consequent artifacts [8].
Bowel preparation before the examination is not routinely suggested. When required, it consists of two doses of an oral laxative the day before imaging, or an enema; the patient should also follow a low-residue dietary regimen on the day before and on the day of the MRI [18].
The supine position is recommended, but for those who suffer from claustrophobia, the prone position is accepted [19].
Just before the MRI, it is recommended to perform an injection of 10 mg or 20 mg of butylescopolamine, an anti-peristaltic agent, to reduce bowel peristalsis. Intravenous administration is preferred to intra-muscular [20].
Vaginal opacification is considered an option in the valuation of deep pelvic endometriosis. Some studies describe a better visualization of small implants in the middle compartment, while others do not find any advantage. Usually, when performed, 20 mL of sonographic gel is infused into the vagina to distend the fornix [12]. Rectal opacification is suggested as an option: some studies consider this practice as useless and uncomfortable for the patient, causing movement artifacts and an increase in bowel peristalsis, which may cause blurring of the images. Moreover, the non-dilated colon above may become spastic. In addition, bowel wall retracting, a valuable sign of the presence of an endometriotic lesion, is likely to disappear when the rectum is distended [20]. Regarding timing, some authors underline the need to perform the exam between the 8th and 12th day of the menstrual cycle so as to obtain a spontaneous T1-weighted imaging (T1WI) signal intensity of blood in the days before the exam [21].
2.2. Technical Requirements and Protocols of Acquisition
A 1.5 Tesla (T) or more MRI system is strongly recommended. Only a few centers have published some interesting analyses using a 3.0 T, but there is still a lack of information on the comparison of the images obtained by the two systems and, therefore, there is no recommendation for using a specific device or another [23].
Pelvic phased array coils give a higher signal-to-noise ratio (SNR) than a body coil; some centers use endocavitary coil in addition to the pelvic array, although this is not convenient in terms of costs and acceptability [24].
The MRI protocol consists of a T2-weighted imaging (T2WI) sequence without fat suppression [7], which is considered the best for detecting pelvic endometriosis implants. At least two orthogonal planes must be performed (sagittal and axial). An axial T2WI acquisition from the renal hila to the pubic bone, allowing the visualization of kidneys and the right iliac fossa, should be recommended. In addition, a thin oblique section 2D T2WI sequence may be used to detect uterosacral and parametrial implants [25].
T1WI MRI sequences with or without fat suppression are recommended for the study of adnexal endometriosis and for active foci of DIE [26,27].
There is no recommendation regarding diffusion-weighted imaging (DWI) sequences, but some authors suggest that it can be performed to differentiate endometriomas from hemorrhagic cysts, with lower apparent diffusion coefficient (ADC) values in the endometriomas [23].
There is no consensus on the use of intravenous contrast agent in the evaluation of DIE, but it is recommended as an “option” in the evaluation of adnexal endometriosis; it can be useful for recognizing abdominal wall endometriosis, or in the differentiation of an endometrioma from a luteal ovarian cyst or tubo-ovarian abscess due to an intense wall enhancement [28,29].
3. Localization
3.1. Superficial Peritoneal Lesions (SUP)
Superficial endometriosis is the most common form of endometriosis and is present in at least 8 out of 10 women diagnosed with endometriosis.
The lesions are located superficially on the peritoneum and across pelvic organs like ovaries and uterine ligaments.
Compared to deep endometriosis, women have less marked symptoms and medical therapy may be sufficient to control symptoms and ensure a good quality of life [37].
Flat lesions are not easy to detect with MRIs, except if they present hemorrhagic components detected as hyperintense on fat-suppressed T1WI or T1WI. When even an MRI is not adequately sensitive to screen superficial endometriosis, diagnostic laparoscopy followed by histological confirmation remains the gold standard [38].
3.2. Deep Infiltrating Endometriosis (DIE)
DIE occurs if the endometrial tissue has penetrated at least 5 mm beyond the surface of the peritoneum. It is the most aggressive form of endometriosis, often associated with a symptomatology that may not benefit from medical therapy alone. DIE is often associated with conditions of infertility. The role of the radiologist and the MRI, in this case, is to provide indications to the surgeon on the location of the lesions and their extension in order to correctly plan surgery.
According to them, the pelvis should be divided into the following nine compartments, with each one containing specific structures:
- -
-
Right and left anterolateral compartments: distal round ligament;
- -
-
Anterocentral: proximal round ligament and bladder;
- -
-
Right and left mediolateral: parametrium, ureter, uterine artery and pelvic wall (external iliac and/or obturator vessels);
- -
-
Mediocentral: torus, proximal uterosacral ligaments, posterior vaginal fornix, rectovaginal septum, anterior mesorectum and external adenomyosis;
- -
-
Right and left posterolateral: distal uterosacral ligaments, sacro-rectal-genital septum and pelvic wall (sacral roots, sciatic nerve, internal iliac vessels);
- -
-
Posterocentral: rectum and rectosigmoid junction.
They also assigned a tenth compartment to extrapelvic lesions (sigmoid colon, cecum, ileum, appendix, extrapelvic ureters, abdominal wall and inguinal regions).
4. Ovarian Endometrioma
The endometrioma, also known as “chocolate cysts”, develops when superficial endometriotic lesions invade the surface of the ovaries. As a result, the blood produced at each menstrual cycle fails to flow properly and accumulates in the context of the ovary, forming a cyst that can grow over time reaching up to 10–20 cm in exceptional cases.
They are often responsible for adhesions and, therefore, dislocation and fixity of the ovaries. The typical symptoms of an endometriosis cyst are pain and dysmenorrhea. In some cases, the presence of cysts interferes with proper ovulation, leading to reduced chances of conception and/or an increased risk of ectopic pregnancy [48,49].
Rupture of an endometrioma is considered a medical emergency with severe pain, dark blood loss from the vagina, fever, nausea, and vomiting.
Treatment can be pharmacological or surgical. Typically, small cysts require a medical approach, while large cysts require surgery [50].
On an MRI, endometriomas appear as cystic lesions with different signal characteristics according to the age of the blood and the type of hemoglobin present.
Generally, they appear hyperintense on fat-suppressed T1WI and T1WI. The hyperintensity on fat-suppressed T1WI helps differentiate endometriomas from dermoid cyst and teratoma, which usually contain fat [51]. On T2WI, a variable signal can be obtained: a hypointense signal can affect variable portions of the cyst, sometimes also presenting a stratification, until a complete loss of the signal. This is called the shading sign and is correlated to the different state of hemoglobin degradation [52]. The T2 dark spot sign refers to hypointense spots in the wall of the cyst due to the presence of macrophages.
Contrast agents may exclude the presence of enhancing nodules that can be indicative of malignant transformation. MRI is also a valuable aid in achieving a proper differential diagnosis between endometrioma and other ovarian cystic lesions, such as mature cystic teratoma, hemorrhagic cyst, cystadenoma, cystadenofibroma and malignant cystic neoplasm.
Mature cystic teratoma is macroscopically characterized by a multicystic mass containing hair, teeth, and/or skin. Occasionally, a solid component known as Rokitansky’s nodule can be observed within it [54,55]. The abundant presence of adipose tissue allows for a differential diagnosis from endometriomas, as a signal reduction will be observed in sequences with adipose signal suppression [56].
Hemorrhagic ovarian cysts (HOCs) develop when bleeding occurs within a follicle or follicular cyst. On an MRI, hemorrhagic cysts exhibit high signal intensity on pre-contrast fat-suppressed T1WI and low signal intensity on T2WI. They do not show enhancement after gadolinium administration [54].
5. Adenomyosis
Adenomyosis is a disease characterized by the presence of endometrial tissue (glands and stroma) in the context of the myometrium. It is much more common in pluripares and in women undergoing surgery of the uterus [60]. Generally, women complain of extremely severe pain during menstruation and suffer from abdominal cramps, abundant and irregular menstruation, fertility problems and adverse pregnancy outcomes [61,62].
Over the years, authors have proposed different classifications that could collate the different facets of adenomyosis. Among the most recent and complete classifications is the one proposed by Kobaiashi et al., which specifically selected five parameters to describe the disease: (1) the affected area (internal adenomyosis or external adenomyosis); (2) the pattern (diffuse or focal); (3) the size; (4) the location; and (5) the concomitance of other localization of endometriosis or other gynecological pathologies [63].
In order to discuss internal endometriosis, it is essential to introduce the concept of the junctional zone. The junctional zone represents the intermediate layer of the myometrium, which typically exhibits a low signal intensity on T2WI sequences, and appears as a continuous structure under normal physiological conditions [64,65]. The thickness of the junctional zone can vary throughout a woman’s life based on factors such as age and hormonal therapies.
The agreement among the authors is that a thickening of the junction zone (>12 mm) serves as an indirect indication of internal adenomyosis. Nevertheless, according to the recent literature, this particular sign cannot be considered conclusive evidence of the disease on its own. It is, therefore, advised to assess two additional parameters for a comprehensive evaluation [68]:
- -
-
The parameter known as “ratiomax” corresponds to the ratio between JZmax and the total thickness of the myometrium, with a value exceeding 40% typically considered an acceptable diagnostic parameter [68];
- -
-
The parameter called “JZ differential” (JZdiff) measures the maximum and minimum thickness difference between the anterior and posterior uterine walls, where a value greater than 5 mm is indicative [68].
The ENDOVALIRM group considers external adenomyosis as part of mediocentral localization of DIE, both in anterior and posterior subtypes. The external adenomyosis corresponds to an extrinsic involvement of the outer myometrial layers. It is more common that external adenomyosis involves the posterior compartment in association with a severe involvement of the rectum–vaginal space [39].
On T2WI, an irregular thickening of the subserosal myometrium is observed, sometimes in association with hyperintense spots that correspond to cystic components. Another fundamental criterion is the presence of blood components, which can be evaluated in the sequences weighed on T1 [69,70].
It is also important for differential diagnosis, even with transient uterine contractions. During uterine contractions, a temporary localized area of decreased signal intensity can occur along with thickening of the junctional zone. This natural process may mimic the appearance of focal or diffuse adenomyosis. If there is uncertainty regarding the nature of a bulge or a region of low intensity in the myometrium, it becomes the responsibility of the radiologist to repeat the imaging after an appropriate interval, typically within 20 to 45 min [72,73].
6. Atypical Site
6.1. Soft Tissues Localization
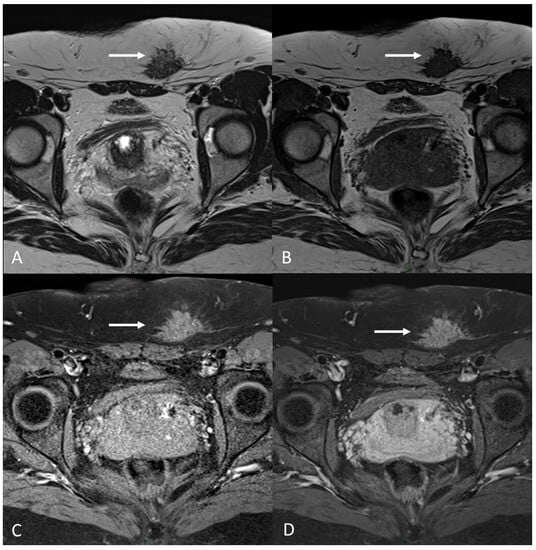
Abdominal endometriosis is often associated with a cutaneous scar from a gynecologic procedure, notably from a cesarean section. Lesions are generally located in subcutaneous soft tissues or inside the muscle layer [79] (Figure 12).

Figure 12. C-section scar endometriosis in a 38-year-old female with subcutaneous nodule causing increasing pain during menstrual cycle. (A,B) Axial T2WI, T1WI. Nodular hypointense lesion with spiculated margins in subcutaneous soft tissues of abdominal wall; (C) Axial fat-suppressed T1WI. The lesion shows small hyperintense foci; (D) Axial T1WI post-contrast medium. The lesion shows homogenous contrast enhancement (white arrows).
The patient often realizes the presence of a mass or an abnormal thickening at the site of the scar. Ultrasound is generally the first level diagnostic test, useful to exclude much more common alterations such as lipomas or hernias, followed by a magnetic resonance exam [80].
MRI increases the specificity for diagnosis while detecting hemorrhagic spots within the fibrotic tissue.
The other atypical site of cutaneous endometriosis is the umbilicus, followed by the inguinal region [81].
6.2. Gastrointestinal Localization
The bowel is the most common extragenital site in about a third of women with DIE. The associated symptoms can be multiple and nonspecific, such as constipation, diarrhea, melena, hematochezia, tenesmus, abdominal discomfort or meteorism. Excluding the rectum and the rectosigmoid junction, the most affected sites are the appendix, cecum and distal ileum. The enteric nodules generally leave the mucosa intact [84].
6.3. Thoracic Endometriosis
Thoracic and pleuric endometriosis are the most frequent extrapelvic manifestations, which are called thoracic endometriosis syndrome (TES). The pleuric disease could manifest as pneumo/hemo-thorax or pneumomediastinum associated with chest pain, dyspnea and sometimes hemoptysis. The pulmonary form of the disease presents as catamenial hemoptysis, pulmonary nodules or diaphragm foci [85].
6.4. Neural Involvement
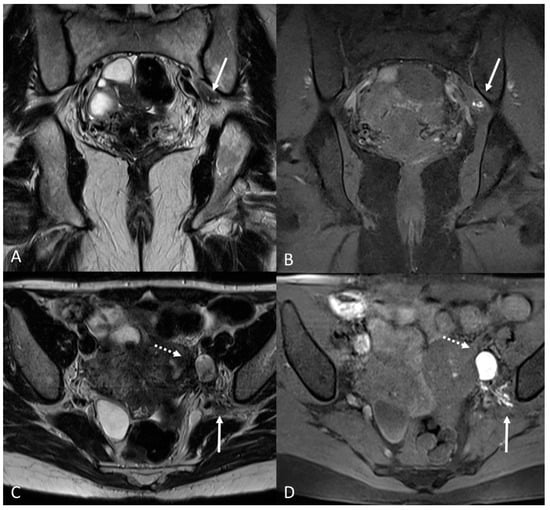
There are also cases of involvement of peripheral nerve plexuses of the pelvic region, such as sciatic, obturator, femoral, and pudendal nerves and their branches (Figure 14).

Figure 14. Localization of the left sciatic nerve and para-uterine endometriotic nodule in a 43-year-olf female with a previously diagnosed endometriosis and surgery approach, accepted at the emergency department for reported left lumbar pain, which radiates to the left side and is associated with episodes of vomiting. (A) Coronal T2WI. Involvement of peripheral nerve plexuses of the pelvic region (sciatic nerve) appearing as a hypointense plaque (white arrow); (B) Coronal fat-suppressed T1WI. The lesion shows small hematic foci (white arrow); (C) Axial T2WI. Para-uterine endometriotic nodule (white dotted arrow) shows variable intensity ranging from low intensity (referred to as shading) to intermediate or high intensity; (D) Axial T1WI. Para-uterine endometriotic nodule with hyperintense signal (white dotted arrow).
7. Post-Operative Imaging
Treatment approaches have to be assessed based on multiple factors.
Medical therapy is definitely the first-line therapeutic option for patients with manageable pelvic pain and no desire for immediate pregnancy. It consists of combined oral contraceptives, progesterone or estrogen. In the second line, analogs of GnRH can be used. Medical therapy also includes specific pain medications and can often be a combination of drugs [87].
The approach is generally laparoscopical with minimally invasive techniques, and it consists of the identification and removal of endometriotic foci and the restoration of the correct pelvic anatomy [35].
The laparotomy approach is reserved for rare cases where lesions are much more extensive.
Post-operative MRI can be useful both to document the success of the surgery with normal post-operative findings and to highlight some paraphysiological conditions such as the presence of fibrosis, adhesions or even alterations of the pelvic anatomy that can contribute to symptoms or disorders.
One of the main challenges for the radiologist is to make a correct differential diagnosis between post-operative fibrosis and recurring/residual disease.
MRI can highlight the difference between these two conditions: fibrotic scars or plaques appear as bands of hypointensity on T2WI, without areas of cystic degeneration, blood foci (hyperintense in T1WI) or even nodular thickenings that are otherwise typical signs of endometriotic lesions [89].
The posterior compartment is the most involved in cases of DIE and therefore often a location of surgical resections.
MRI after surgery may show an alteration of the physiological morphology of the pelvis due to the presence of adhesions and sometimes because of the obliterations of anatomical spaces and pouch without a fat tissue interface between the bladder, uterus, vagina and rectum [91].
Thinning of the uterine or vaginal wall may also be observed; this alteration can lead to an increased risk of perforation or rupture of the aforementioned organs.
The surgical management of intestinal endometriosis is a prerogative of both gynecology and general surgery. A careful pre-operative evaluation is essential to plan the best possible surgery, in order to be as radical as possible without compromising the quality of life of patients [92].
The presence of free intraperitoneal fluid or gas associated with severe symptomatology may indicate post-operative complications such as perforation or fistula.
Surgery for the treatment of endometrioma should also be mentioned.
8. Conclusions
For the diagnostic process, it is fundamental to have an adequate protocol in order to detect the lesions, including a T2-weighted sequence in at least two orthogonal planes (sagittal and axial), a T1-weighted sequence with or without fat suppression to detect adnexal endometriosis and active foci of deep infiltrating endometriosis, and a T2-star-weighted imaging sequence for the detection of hemosiderin.
Furthermore, the evolving role of AI in the diagnosis of endometriosis is a promising avenue. In fact, it could be a valuable tool to assist radiologists in identifying and characterizing lesions, showcasing the substantial impact on clinical practice in terms of accuracy and efficiency.
This entry is adapted from the peer-reviewed paper 10.3390/app131810509
This entry is offline, you can click here to edit this entry!
