Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
To feed the ever-increasing population under changing climate scenarios, it is imperative to investigate the role of halophytes, which are equipped with special adaptation mechanisms to cope under extreme conditions of salinity. A systematic approach was developed that deciphers those metabolites involved in regulating the physiological, biochemical, and molecular responses of halophytes to salt stress.
- climate change
- rhizosphere microbes
- secondary metabolites
- halophytes
1. Impact of Climate Change on Biosaline Agriculture and the Role of Halophytes
Current climate change poses a serious threat to agricultural productivity. A rise in the atmospheric CO2 level, heat waves, elevated temperature, soil drought, and salinity are all major outcomes of climate change that adversely affect the growth of glycophytes [1,2]. Halophyte crops are better alternatives for food, fodder, fiber, fuel, essential oil, and medicines, as they have developed special characteristics to cope with environmental extremes. Halophytes possess a greater degree of tolerance than non-halophyte crops. Some parasitic plants develop succulence when growing on halophytic hosts, and an interesting finding is that halophytes growing in their natural habitats do not show signs of oxidative stress [3]. Halogeton glomeratus is an annual herbaceous and succulent halophyte belonging to the family Chenopodiaceae. It is considered a potential source for oilseed production and also assists in phytoremediation. Exposure of H. glomeratus to long-term salinity and drought stress results in decreased chlorophyll and carotenoid content and inhibition of the photosynthetic rate, transpiration rate, stomatal conductance, water potential, and biomass [4].
Halophyte plants survive in soil where salinity is around 200 mM NaCl [5]. Plant ecologists classify halophytes into three main groups. The first is euhalophytes that dilute salt within their stems or leaves and have a strong capability to endure salt stress. Recretohalophytes secrete salt from their leaves and grow widely around the world, inhabiting inland saline lands and seawater. Pseudo-halophytes can not only hold up ions in roots but also minimize their transport to the shoot parts. On the basis of morphology, they are classified into two groups: excretive and succulent. Based on salt demand and tolerance to NaCl, they can be classified as obligate halophytes, facultative halophytes, and habitat-indifferent halophytes. Obligate halophytes are true halophytes, as they need salt for their growth. Members of the family Chenopodiacea belong to this category. Facultative halophytes generally grow in soil where salts are in quite low concentrations, but they can also grow under saline conditions. Monocots belonging to families Poaceae, Cypraceae, and Juncaceae and a large number of dicotyledons belong to this group. Habitat-indifferent halophytes prefer to live in saline-free soil but can also thrive in saline soil. Examples are Festuca rubra, Agrostis stolonifera, and Juncus bufonius. On the basis of habitat, halophytes are classified into hydrophalophytes (grow in saline water or salt marshes) and xerohalophytes (grow in desert). Salinity induces the release of oleanolic acid in the root exudates of Salicornia, which act as chemoattractants for colonizing halophilic siderophores producing Halomonas anticariensis FP35T [6]. In return, colonization of this bacterium enhances the positive effects on the root length, shoot length, germination, and vigor index of S. hispanica [6].
2. Halophytes as Crop Plants
For agricultural sustainability under saline conditions, two possible approaches are needed: (i) improving salt tolerance of cultivated crops, or (ii) domestication of halophytes [5]. The majority of crop plants are non-halophytes. Genetically modified crops have been developed for biosaline agriculture, but this is time consuming and involves many genes possessing various pros and cons and causes various allergic reactions, disrupting natural gene flow and increasing the risk factors of human diseases [7].
The cultivation of halophytes for food, fodder, edible oil, biofuel, and medicine seems to be an alternate option, as they are fully equipped with better salt tolerance properties. Many species of halophytes have the potential to be used as gourmet vegetables and salad, i.e., Aster tripolium, Atriplex hortensis, Beta maritime, Crambe maritima, Crithmum maritimum, Inula crithmoides, Salicornia spp., Salsola soda, and Tetragonia tetragonioides. Some wild edible halophytes are in the genera Bassia, Beta, Cakile, Chenopodium, Plantago, Portulaca, and Suaeda. Atriplex halimus, Salicornica fruticosa, and Cakile maritime accumulate Na+ in their leaves, but the content of this ion is lower compared to other culinary halophytes. These plants can be used as sources of green salt (plant-based salt contains 50 % less Na than common salt) due to their nutritional value [8,9]. The seeds and lignocellulosic biomass of several halophyte genera, i.e., Salicornia, Suaeda, Atriplex, Distchlis, and Batis, have been exploited for the production of biofuel (bioethanol). Suaeda is a high salt tolerant C4 species. This genus ameliorates contaminated saline soil and is also used in food, fodder, medicine, and bioenergy.
A wide group of halophyte species (2500–3000) occur naturally in salt marshes, providing an opportunity to use them as crop plants to combat food security in the future. Seven species of plants, namely Suaeda glauca, Bassia scoparia, H. glomeratus, Kalidium foliatum, Medicago falcata, Atriplex canescens, and Artemisia desertorum, can be used as enrichment materials for Zn and Cu. H. glomeratus has medicinal value in traditional Chinese medicine. Wang et al. [10] identified secondary metabolites that included flavones, flavonols, flavandiols, glucosinolates, isoquinolines, pyridines, indoles, amino acids, lipids, carbohydrates, and ATP-binding cassette transporters. These metabolites regulate osmotic adjustment and modulate the adjustment of membrane lipid action in H. glomeratus. These metabolites also have applications in human cardiovascular diseases, cancers, diabetes, and heart diseases.
3. Salt Tolerance Mechanism of Halophytes: Role of Secondary Metabolites
According to Meng et al. [27], halophytes have adapted to thrive under high salinity conditions by secreting salt crystals through salt glands, regulating cellular ion homeostasis and osmotic pressure, detoxifying reactive oxygen species (ROS), and bringing alterations in membrane composition. A diverse group of secondary metabolites is involved in regulating these functions. Functionally, they are classified as osmoprotectants, antioxidants, polyamines, and phytohormones. On the basis of their biosynthetic pathway, they are categorized into (i) phenolic groups (composed of single sugar and benzene rings); (ii) terpenes and steroids; and (iii) N-containing compounds.
4. Halobacteria Diversity
Halobacterium species are obligate aerobic, rod-shaped archaea enveloped by a single lipid bilayer membrane surrounded by an S-layer made from the cell-surface glycoprotein [7]. They can use amino acids for their growth in aerobic conditions, but they can also grow in an anaerobic environment, given the correct conditions [7]. Halobacteria can be found in highly saline lakes, such as the Great Salt Lake, the Dead Sea, and Lake Magadi. Halobacteria are candidates for a life form present on Mars. These microorganisms develop a thin crust of salt that can moderate some of the ultraviolet light and make it opaque through their photosynthetic pigment bacteriorhodopsin [7].
Halobacteria are able to survive under a wide range of salinities. According to their survival potential, they are classified as halotolerant or halophilic bacteria. Halotolerant bacteria can survive in media up to 25% NaCl, whereas halophile bacteria require salt to grow. They have been isolated from different halophyte plants across the world, i.e., the rhizosphere of Leymus chinensis, Puccinellia tenuiflora, and Suaeda glauca, are highly enriched with the phyla of bacteria Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Gemmatimonadetes, Haloarchaea, and Proteobacteria [49,50,51]. The Firmicutes phylum consisted of genera Bacillus, Virgibacillus, Salincoccus, Marinococcus, Halobacillus, Planococcus, Thalassobacillus, and Salimicrobium; in phylum Actinobacteria, Nocardiopsis was the representative genus; in phylum Proteobacteria, Halomonas, Idiomarina, and Psychrobacter were representative genera [48]. Halobacteria exhibit a highly complex network of diversity than endophytic bacteria and bacteria from bulk soil. Gao et al. [52] reported Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in the rhizosphere of Salicornica europaea, Kalidium foliatum, and Borsczowia aralocaspica. These bacteria exhibit a variety of mechanisms that have multiple roles, e.g., safeguard halophytes from salt stress, promote their growth, and remediate soil contamination (Figure 1).
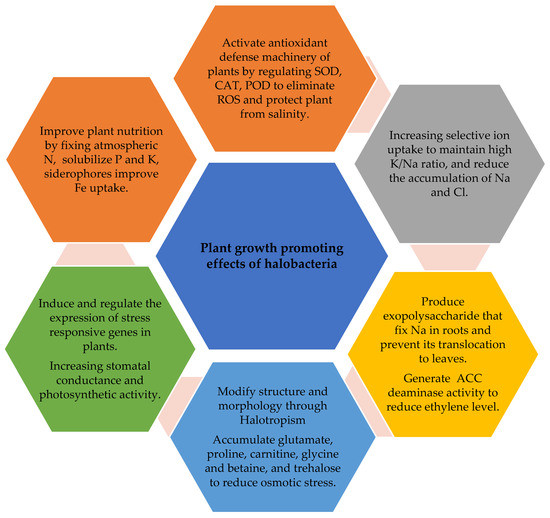
Figure 1. Mechanism of halobacteria in ameliorating salt stress and improving growth and yield of halophytes under saline conditions.
5. Climate Change Modulates Rhizosphere Microbial Community
The main factors governing the diversity of the rhizosphere microbiome are (1) climatic factors (precipitation, temperature, salinity, drought, etc.), (2) soil physicochemical properties (pH, EC, cation exchange capacity, organic C content), and (3) root exudation pattern and composition [45]. Lashini et al. [53] isolated rhizobacteria from the rhizosphere soil of olive trees grown in semiarid and arid areas of Morocco. The strains were 85% halotolerant and 65% thermotolerant, and they were able to overcome high salinity (≥4%) and temperature stress (≥45 °C). They were identified as Bacillus licheniformis, Arthrobacter globiformis, and Bacillus megaterium. About 21% of photosynthetically fixed C is transferred into plant roots and root exudates in the form of soluble sugars, phenolic acids, amino acids, and organic acids. The elevated level of such compounds alters the community composition and structure of active bacteria [54]. The greater input of liable C via root exudates may increase microbial N demand, as competition occurred between microbes and plants for available N. Thus, N dynamics are likely to change under elevated CO2. It also affects the community of N-fixing bacteria [54]. Salinity influences the structure of the rhizosphere microbiome of halophytes. Mukhtar et al. [55] conducted a study to compare the composition of the rhizosphere microbiome of halophytes Urochloa, Kochia, Salsola, and Atriplex inhabiting the moderate and high saline environment of Khewra salt rang, Pakistan, with that of non-halophyte Triticum. Analysis of the 16S rRNA gene showed that Actinobacteria were dominant in the saline soil. Other identified groups were Euryarchaeota, Ignavibacteriae, and Nanohaloarchaeota. Soil physicochemical properties, i.e., pH, EC, and SOC, also influence the microbe community. For example, TS, Cl−, SO42−, HCO3−1, Na+, and Mg+ are the main factors influencing the rhizosphere microbiome structure [56]. Soil pH is one of the key factors shaping halophytic rhizosphere soil bacterial community composition and diversity, and SWC content is a possible factor affecting bacterial community functions [57].
6. Secondary Metabolites Recruit Beneficial Rhizospheric Microbes
Root exudates are crucial in modulating the composition and functional diversity of rhizosphere microbes. Mucilage is actively released from the roots, while diffusates are passively released due to osmotic differences between the soil solution and the cell. Both types include organic compounds, which are classified into low and high molecular weight compounds. Heavy molecular weight compounds (i.e., mucilage, cellulose) are not easily used by microbes and make up the majority of C released from roots. Low molecular weight compounds are highly diverse and have a wide array of functions. These consist of organic acids, amino acids, proteins, sugar, phenolics, and other secondary metabolites (including benzoxazinoids, coumarins, flavonoids, indole compounds, and terpenes) that shape the rhizosphere microbiome [58].
Plant secondary metabolites have differential effects on soil microbiota. Two indole metabolites, benzoxazolinone and gramine, produced by different Graminae species, and quercetin, a flavonoid synthesized by many dicot species, revealed significant effects on soil bacteria, with benzoxazolinone showing a predominantly inhibitory effect preventing the accumulation of many predominantly harmful taxa, while gramine and quercetin mostly exert their function by attracting beneficial bacteria [58]. Oleanolic acid possesses chemoattractant properties and is the principal constituent of Salicornia root exudates that have the potential to expand the colonization of salt-tolerant Salicornia hispanica by halophilic siderophore-producing bacteria Halomonas anticariensis FP35T [6].
Terpene is a highly diverse group of secondary metabolites that play an important role in plant microbe interaction. Arabidopsis thaliana root exudates contain triterpenes, namely thalianin, thalianyl medium-chain fatty acid esters (three steps), and arabidin, that are involved in belowground communication and able to directly modulate A. thaliana-specific root bacterial communities in a very selective manner [59]. Xiong et al. [60] reported that organic acids (2-methylbutyric acid, stearic acid, palmitic acid, palmitoleic acid, and oleic acid) found in the root exudates of Limonium sinesis promoted the chemotaxis of the rhizosphere PGPR strain Bacillus flexus KLBMP 4941. Strigolactone is a new class of phytohormone that acts as a signaling molecule in symbiosis to recruit root- and rhizosphere-associated microbiomes. Kim et al. [61] analyzed the bacterial and fungal microbial communities of 16 rice genotypes differing in exudation of root strigolactone. The results showed that structural differences in the exuded strigolactones affected different sets of microbes, i.e., the relative abundance of phosphate solubilizing microbes; Burkholderia, Caballeronia, Paraburkholderia and Acidobacteria were linked to orobanchol strigolactone, whereas 4-deoxyorobanchol was associated with genera Dyella and Umbelopsis [61].
7. Halobacteria for Biosaline Agriculture
Halobacteria possess plant growth promoting characteristics, i.e., Reang et al. [62] isolated halotolerant and halophilic bacteria belonging to Halomonas pacifca, H. stenophila, Bacillus haynesii, B. licheniformis, and Oceanobacillus aidingensis. These isolates were able to produce indole acetic acid, solubilized phosphate, and potash and showed N-fixing capacity. There is a growing number of publications on plant growth promotion by halophilic rhizobacteria isolated from the roots of halophyte species [63,64].
Endophytic halophiles (Halomonas and Bacillus) isolated from the roots of halophiles showed maximum salt tolerance up to 4 mM NaCl [65]. When these isolates were used to inoculate alfalfa seedlings, they stimulated plant growth in the presence of 1% NaCl, a level that significantly inhibited the growth of uninoculated plants. Exopolysaccharides (EPS) are commonly produced metabolites by halotolerant bacteria, which exhibit 40–90% extracellular matrix of bacteria under stress conditions [66]. EPS exhibits antioxidant activity and confers tolerance to bacteria against reactive oxygen species. This property can be exploited to alleviate salt stress damage in crops [67].
Robinia pseudoacacia seedlings exposed to VOCs of the JZ-GX1 strain showed increases in biomass, plant development, and lateral root numbers. Additionally, decreases in malondialdehyde, superoxide anion (O2−) and hydrogen peroxide (H2O2) contents and increases in proline contents and superoxide dismutase, peroxidase, and glutathione reductase activities were observed in Acacia leaves. Notably, the sodium–potassium ratio in the roots, stems, and leaves of Acacia exposed to VOCs of the JZ-GX1 strain were significantly lower than those in the control samples [68]. Swiss Chard inoculated with halotolerant PGPR and watered with 85 nmol L−1 NaCl showed higher values of leaf dry weight than control plants [69]. Furthermore, PGPR inoculation reduced electrolyte leakage and Na+ uptake and improved the chlorophyll a fluorescence parameters, chlorophyll, and carotenoid concentrations, stomatal conductance, and antioxidant capacity of Swiss chard. Nijafi Zilaie et al. [70] reported that two halotolerant bacteria Bacillus pumilus and Zhihengliuella halotolerans were able to reduce the content of ascorbic acid, flavonoid, total phenol, proline, malondialdehyde, and catalase activity, and ultimately improved the antioxidant capacity of Haloxylon aphyllum.
This entry is adapted from the peer-reviewed paper 10.3390/app13031299
This entry is offline, you can click here to edit this entry!
