Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Autoimmune thyroid diseases are on the rise worldwide, and such a rapid increase is mainly driven by environmental factors related to changed lifestyles in “modern” societies.
- autoimmune thyroid diseases
- Hashimoto’s thyroiditis
- Mediterranean diet
1. Introduction
Autoimmune thyroid diseases (AITDs) include a wide spectrum of disorders, ranging from hyperthyroidism and goiter (Graves’ disease, GD) to hypothyroidism and glandular hypo-atrophia (Hashimoto’s thyroiditis, HT) [1,2]. They are the result of a failure of immunological tolerance to self-antigens and consequent activation of immune responses against thyroid tissue. AITDs are multifactorial diseases, deriving from the interaction between genetic and environmental factors, which trigger the development and further progression of AITDs in genetically susceptible individuals [1,2,3,4]. GD is an uncommon disease, and its prevalence/incidence has remained stable over the years [5,6], whereas autoimmune thyroiditis (namely, HT), is very common, affecting about 3–5% of the general population, and its incidence has been increasing [7,8,9]. In recent decades, large epidemiological studies have reported increased prevalence and incidence of several autoimmune disorders, including rheumatic (systemic lupus erythematosus, rheumatoid arthritis, to mention a few), neurological (multiple sclerosis, myasthenia gravis), gastro-enteric (inflammatory bowel diseases, celiac disease) and endocrine (such as autoimmune thyroiditis, type 1 diabetes) diseases, mostly in developed countries of the West and North compared to developing countries of the East and South [10,11,12].
Such an increase clearly correlates with improved hygiene and health standards, as well as with socioeconomic status, suggesting that environmental factors are driving these geo-epidemiologic changes [1,10,11,12]. In particular, interest has been increasing in the “modern” lifestyle of industrialized, “westernized” societies, and to the potential environmental triggers that characterize it, including, for instance, reduced microbial exposures and improved hygiene, pollutants, psychological stress overload, deficiencies or excess of nutrients (notably, vitamin D, iodine, and selenium, to mention a few), whose role in autoimmunity is intensely debated [12,13,14,15,16,17,18,19,20]. In particular, in developed countries, excess calorie intake and frequent consumption of “unhealthy food”, associated with a sedentary lifestyle, has led to a rise in the prevalence of obesity, which in turn predisposes individuals to several chronic non-communicable diseases, including inflammatory and immune-mediated disorders [19,20,21,22]. For this reason, interest in nutritional patterns has grown in recent years and our understanding of the link between diet and thyroid function is rapidly expanding.
2. Dietary Habits and Thyroid Autoimmune Diseases
2.1. Evidence from Clinical Studies
The association between diet and risk of developing autoimmune disorders was proposed more than 50 years ago by Trowell, who observed that autoimmune disorders, including thyroiditis, were extremely rare among isolated rural sub-Saharan populations following traditional near-vegan diets [62,63]. A similar low incidence of autoimmune disorders was reported in Asian societies whose diets were almost vegan [64,65]. In the following decades, evidence has been growing on the role of diet in the development of several autoimmune disorders, including rheumatoid arthritis, celiac and intestinal bowel diseases, type 1 diabetes, multiple sclerosis, and psoriasis [21,22,23,24,25,66,67,68,69,70,71].
In 2015, Tonstad and coworkers investigated the association between incident and/or prevalent hyperthyroidism and dietary patterns among a large cohort of subjects belonging to the Seventh-day Adventist church, who exhibited a wide range of diets from vegan to omnivorous, with a high proportion of vegetarians. The authors observed that the prevalence of hyperthyroidism was significantly lower in subjects following a vegan diet compared to omnivores, while lacto-ovo and pesco-vegetarian diets were associated with intermediate protection [72]. Overall, this study suggested that diets excluding animal foods could be protective against thyroid dysfunction, likely autoimmune in etiology [72]. Even if the MD was not specifically studied in this case, and even if the MD does not completely exclude animal-derived products, these results can be considered relevant for our discussion. Indeed, it must be remembered that in MD, most of food intake is derived from whole grains and vegetables, with a much lower content in animal-derived food compared to the WD. Several recent studies focused on the effect of dietary patterns on thyroid function parameters. Zupo et al. investigated the possible relationship between adherence to the MD, assessed through the PREDIMED questionnaire, and thyroid function in 324 euthyroid overweight/obese subjects living in Southern Italy [73]. They found that a higher adherence to the MD was inversely related to serum levels of free T3 (fT3) and free T4 (fT4). When considering the single items of the PREDIMED questionnaire, it emerged that a higher consumption of extra-virgin olive oil was associated with lower levels of fT3 and fT4. A logistic regression model adjusted for gender, age, and BMI confirmed only the correlation between FT4 and adherence the MD, whereas no effect on serum TSH was observed [73]. The study by Liu and coworkers aimed at investigating the association between dietary inflammatory potential and thyroid function in a large population of adult males (2346 subjects), using data from the National Health and Nutrition Examination Survey [74]. The dietary inflammatory index was calculated based on the reported intake of several food groups that have anti-inflammatory potential (including fiber, vitamin C, flavonoids, garlic, several spices like rosemary and thyme) vs. pro-inflammatory ones (such as animal fat, carbohydrates, and animal protein). The results showed a positive association between dietary inflammatory index and total T4, and subjects adhering to a more pro-inflammatory diet appeared to have higher total T4 and total T3 levels, even within the normal range, while no consistent effect on fT3, fT4, or TSH could be observed. The relationship appeared to be stronger among obese subjects and those with urinary iodine concentration (UIC) levels indicating iodine deficiency [74]. The interpretation of these two recent studies performed on thyroid function parameters and dietary patterns can be cumbersome, due to the multiple factors that can influence circulating thyroid hormones, especially with normal TSH. The lack of a strong observed effect of the different dietary choices on TSH levels, and the fact that the variation in circulating T3 and T4 is of small entity and within the normal range seems to suggest that these subjects do not experience hypothyroidism, but rather small modifications in peripheral sensitivity to thyroid hormones and/or binding of thyroid hormones with transport proteins.
In 2020, Kaličanin and coworkers evaluated the differences in food-group consumption between HT patients and healthy subjects as controls through a food frequency questionnaire (FFQ) [75]. A specific focus was put on fat consumption, in particular regarding the choice between vegetal and animal fats. The results showed that HT patients consumed more animal fat and processed meat than controls, who in turn consumed more non-processed red meat, non-alcoholic beverages, whole grains, and plant oils (Table 1). A subgroup analysis showed that HT patients on levothyroxine (LT4) therapy consumed more red meat than those who were not receiving substitutive therapy [76]. Moreover, from analysis of food consumption, it emerged that HT patients do not modify their dietary habits upon disease diagnosis, indicating that nutritional aspects were disregarded by both the patients and the physicians [75]. A more recent study by Ruggeri et al. evaluated the dietary habits of a cohort of euthyroid subjects from Southern Italy and found significant differences between HT patients and healthy subjects [37]. In particular, HT subjects consumed higher amounts of animal-derived food, including meat (both fresh and processed), dairy, and fish, and commercial sweetened products, while controls reported higher intake of vegetables, legumes, and nuts (Table 1). Of note, HT subjects and healthy controls did not differ in terms of either body weight or BMI, most of them being of normal weight. Adherence to the MD, as assessed by PREDIMED questionnaire, was lower in HT patients compared to controls, and in a multivariate logistic regression model, the PREDIMED score was an independent predictor of thyroid autoantibodies positivity, suggesting a protective role of the MD against thyroid autoimmunity [37].
Table 1. Summary of studies (either clinical or experimental) evaluating the relationship between dietary habits and thyroid functional status and/or autoimmunity.
| Study | Dietary Habits | Design of the Study | Thyroid Effects |
| Tonstad et al. [72] |
Vegetarian diets vs. omnivorous diets |
Observational clinical study on 65,981 subjects, members of the Seventh-day Adventist church | Lower risk of prevalent hyperthyroidism in vegan (OR = 0.49; 95% CI 0.33–0·72), lacto-ovo (OR = 0.65; 95% CI 0.53–0.81), and pesco-vegetarian (OR = 0.74; 95% CI 0.56–1.00) diets than in omnivorous diets |
| Zupo et al. [73] |
Adherence to the MD (PREDIMED score) EVOO consumption |
Observational study on a cohort of 324 euthyroid overweight/obese subjects (228 F and 96 M, aged 14–72 years) |
Inverse relation with serum fT3 (p < 0.01) and fT4 (p < 0.01) levels; no effect on serum TSH |
| Liu et al. [74] |
Dietary inflammatory potential (DIP) score |
Cross-sectional study including 2346 U.S. male subjects aged ≥ 20 years (data from NHANES) |
Positive association with serum TT4 (β = 0.07; p = 0.0044); no effect of serum fT3, fT4 or TSH |
| Kaličanin et al. [75] |
Food group consumption frequency |
Observational study including 491 HT patients and 433 controls |
↑ consumption of animal fat (OR 1.55, p < 0.0001) and processed meat (OR 1.16, p = 0.0012) in HT pts. ↑ consumption of red meat (OR 0.80, p < 0.0001), non-alcoholic beverages (OR 0.82, p < 0.0001), whole grains (OR 0.82, p < 0.0001), and plant oil (OR 0.87, p < 0.0001) in controls Association of plant oil consumption with increased fT3 levels in HT patients (β = 0.07, p < 0.0001) |
| Ruggeri et al. [37] |
Food group consumption frequency Adherence to the MD (PREDIMED score) |
Observational study including 81 (71 F, 10 M) HT patients and 119 (102 F, 17 M) controls | ↑ intake frequencies of animal foods (meat, p = 0.0001; fish, p = 0.0001; dairy products, p = 0.004) in HT pts ↑ intake frequencies of plant foods (legumes, p = 0.001; fruits and vegetables, p = 0.030; nuts, p = 0.0005) in controls Lower adherence to the Mediterranean diet in HT patients compared to controls (p = 0.0001) PREDIMED score was a predictor of TPOAb positivity (OR 0.192, 95% CI 0.074–0.500, p = 0.001) |
2.2. Pathophysiological Bases of the Link between Dietary Components and Thyroid Autoimmune Diseases
The pathophysiological mechanisms underlying the relationship between MD, WD, and thyroid autoimmunity are not completely understood, but some hypotheses can be made, and the possible role of the various food components can be discussed, as exemplified in Figure 1.
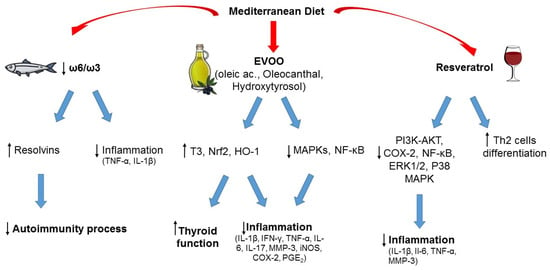
Figure 1. Typical foods from Mediterranean diet and their possible role in the prevention of autoimmunity and inflammation. ↑ increased; ↓ decreased.
2.2.1. Animal Products
The lower consumption of animal-derived products in subjects adhering to the MD when compared to the WD appears to have a central role in protecting against thyroid autoimmunity and/or dysfunction, as suggested by pre-clinical studies [76,77,78]. Among the possible mechanisms that could be involved in this association, it could be hypothesized that high intake of animal fat could increase the risk of autoimmunity due to an increase in ROS production. Indeed, in a 2016 study by Ruggeri et al., a higher consumption of animal-derived products in patients with TH was related to a higher concentration of oxidant factors and a lower concentration of antioxidants [37]. Another reason that could explain the effect of meat consumption, especially if processed, on thyroid autoimmunity is the intake of nitrates and nitrites. Nitrites can be found in several processed foods, in particular industrial processed meat. Indeed, exposure of laboratory animals to extremely high concentrations of nitrites (~10–600 times more than the acceptable daily intake) can induce several anti-thyroid effects, including reduced levels of circulating thyroid hormones and histomorphological changes in thyroid gland. However, it should be acknowledged that no such effects have been documented in humans [79].
The role of the other main source of animal-derived food, that is fish, is more controversial in terms of effect on thyroid function and autoimmunity [37,50,80]. The MD promotes fish consumption rather than meat. Fish represents the main dietary source of omega-3 PUFAs, whose positive effects on chronic inflammatory and immune-mediated disorders are well known (see also below). On the other hand, the risk of fish contamination by environmental pollutants should be not overlooked, and a cost (contaminants) to benefits (healthy components) ratio should always be considered [50,80]. Of note, the MD is characterized by prevalent consumption of small oily fishes (the so-called “blue fish”, like anchovies, sardines, mackerels, etc.), that represent a good source of proteins and PUFAs and are associated with a low risk of contamination, whereas large, top-predator fishes (like swordfish) tend to concentrate pollutants, in particular heavy metals, persistent organic pollutants, and microplastics, due to mechanisms of bioaccumulation and biomagnification [50,80]. Several studies suggest that consumption of PUFAs, in particular omega-3, can have protective effects on the development of autoimmune thyroiditis, while heavy metal exposure was associated with an increased risk of autoimmunity [50,80]. Thus, the kind of fish consumed seems to play a role, rather than consumption of fish per se. When including fish with low content of contaminants, the MD represents a healthy nutritional model, because fish is part of a complete healthy dietary plan, and patients would benefit from both fish intake and other micronutrients [50].
2.2.2. Monounsaturated and Polyunsaturated Fatty Acids (Omega-3)
Linoleic acid and alpha-linolenic acid are essential fatty acids, as their intake totally depends on foods because they cannot be synthesized in the human body, and represent the precursors of ω-6 and ω-3 PUFA families. Besides alpha-linolenic acid, the other two main ω-3 PUFAs, directly derived from it, are eicosapentaenoic acid and docosahexaenoic acid. The main difference between the two classes of PUFA is the position of the double bonds on the carbon chain (the ω-carbon): ω-6 PUFAs have the first double bond at the sixth carbon, while ω-3 PUFAs have it at the third carbon starting from the methyl end of the carbon chain. ω-6 PUFAs can be found in vegetable oils (soybean, corn, sunflower oils) in the form of linoleic acid, and animal products, whereas green leafy vegetables are a major source of ω-3 PUFAs in the form of alpha-linolenic acid. Fish and fish oil provide a good supply of eicosapentaenoic and docosahexaenoic acid [81]. Both ω-6 and ω-3 PUFAs are precursors of inflammatory mediators but with opposite effects: ω-3 PUFAs reduce inflammation whereas ω-6 PUFAs tend to promote inflammation. For this reason, a balanced ω-6/ω-3 ratio is important to support the anti-inflammatory profile of ω-3 PUFAs, and a varied diet that includes balanced amounts of each PUFA class has prevalent anti-inflammatory effects. Different from the WD, which is characterized by an excessive ω-6 PUFA intake associated with a higher production of inflammatory cytokines [46,82], the MD ensures high consumption of ω-3-rich food promoting a better inflammatory profile [46].
Dietary ω-3 PUFAs can reduce inflammation through different mechanisms, for example by lowering the synthesis of cytokines (such as TNF-α, IL-1β), prostaglandins, and leukotrienes; by controlling leucocyte chemotaxis, adhesion molecule expression, and leucocyte–endothelial adhesive interactions [48,61]. In recentyears, new classes of lipid mediators have emerged as key players in the resolution of inflammatory process. They are endogenously produced during inflammation from PUFAs and exert potent anti-inflammatory actions, serving as specialized pro-resolving lipid mediators [83]. Bioactive metabolites from ω-3 PUFAs, such as resolvins, maresins, and protectins, demonstrated beneficial effects on global health. Among them, resolvins have a well-documented anti-inflammatory activity [84]. Increasingly, evidence has demonstrated a major role of these metabolites in the regulation of autoimmune processes [61,85].
2.2.3. Extra Virgin Olive Oil (EVOO)
Among the central foods of MD is EVOO. With more than 200 different bioactive compounds detected, including phenolics, sterols, carotenoids, and triterpenic alcohols, it represents the main fat used in the MD and is a hallmark of this dietary pattern. The phenolic composition of EVOO has been associated with its strong antioxidant activity, and it is thought to be largely responsible for the pleiotropic positive effects of the MD [44,86]. Recently, interest has grown in investigating the potential advantages of EVOO consumption in the context of autoimmunity. In several experimental models of autoimmune disease, EVOO components, namely hydroxytyrosol and oleic acid, and oleocanthal, were revealed to be efficacious in counteracting oxidative and inflammatory imbalances by enhancing Nrf2/HO-1 and inhibiting of MAPKs/NF-κB signaling pathways [87,88,89,90]. In two studies, the EVOO-diet supplementation was compared with a sunflower oil-diet supplementation, and both investigations reported greater EVOO effectiveness, probably due to its phenolic composition [89,90]. Olive derivatives showed varying effects on circulating thyroid hormone levels in animal models (both euthyroid and with experimentally induced autoimmune thyroiditis), with an increase in total T3 levels as the most frequently observed one [91]. In a review by Pang et al., the analyses of the literature demonstrated that olive oil, olive leaf extracts, and solid olive residue enhanced thyroid function in both euthyroid and hypothyroid animals, and in the latter ameliorated the oxidative status [91]. Obviously, further studies are required, and these results need to be confirmed in humans. Overall, the results from these studies are encouraging and provide preliminary evidence for the positive value of an EVOO-rich diet like MD to counteract and prevent autoimmune diseases.
2.2.4. Phenolic Compounds of Wine (Resveratrol)
A moderate wine intake, especially red wine, with meals, is considered another unique habit in the context of the MD, with beneficial health effects related to its phenolic composition [92]. Indeed, wine is rich in phenolic compounds derived from the grapes during the wine-making processes. Resveratrol, a stilbene localized in grape skin, is acknowledged as an anti-cancer, anti-inflammatory, neuroprotective, and antioxidant bioactive compound [93,94,95]. Recently, several experimental studies evidenced a potential protective effect of this compound in different autoimmune settings, through the regulation of antioxidants, inflammatory mediators, and Th cell subpopulation balance [96,97,98,99]. Interestingly, a moderate consumption of alcohol was associated with a reduced risk of HT and hypothyroidism in the study by Carlé et al. [100]. The positive effects derived from the consumption of wine are currently under debate, but we must focus on a moderate wine consumption during meals as usually done in the MD habits. Epidemiological and clinical studies, and observations on Mediterranean cohorts of subjects, have demonstrated that moderate wine consumption correlates with benefits to human health [101]. Our intent here is not to promote alcohol as a health element or to induce people to drink alcohol in the hope of gaining benefits, rather to emphasize the role of moderate alcohol consumption, particularly red wine, within the MD and how there are no results to support complete abstention, in order to reduce the risk of developing autoimmune diseases.
This entry is adapted from the peer-reviewed paper 10.3390/nu15183953
This entry is offline, you can click here to edit this entry!
