People around the world are living longer. In 2021, the World Health Organization (WHO) estimated that by 2030, one in six people will be 60 years of age or older. The number of people aged 80 years or older will triple from 2020 to 2050, reaching 426 million [
1]. The epidemiological evidence has demonstrated the effects of age-related challenges, especially in terms of the burdens on economic growth and healthcare systems. These systems are currently facing the challenges of complex health conditions and age-related diseases [
2,
3]. Aging is characterized by the progressive decline in physical and psychological capacities that occurs concomitantly with the onset of chronic degenerative diseases. In this context, the search for molecular targets that could serve as more effective adjuvants in anti-aging therapies or in the treatment of age-related diseases is becoming the number-one priority. The main age-related diseases are cardiovascular, neurodegenerative, and musculoskeletal diseases; arthritis; and cancer [
4]; furthermore, according to the Global Burden of Disease, Injuries, and Risk Factors Study in 2017, 31.4% of all diseases were found to be associated with age [
5]. Interestingly, in recent years, a plethora of cellular and molecular hallmarks of aging have been described, such as genomic instability, a loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, and altered intracellular communication, among others [
6,
7]. Organ and tissue analyses of these molecular changes might provide accessible markers of aging stages and progression and help to identify novel geroprotective compounds. Possible clinical interventions and treatments include strategies such as lifestyle interventions (diet, exercise, and weight loss), pharmacological approaches (antioxidants and senolytic, senomorphic, and anti-inflammatory drugs), modulating the gut microbiota, cell transplantation, gene therapy, and immunotherapy [
8]. All of these strategies have the final goal of achieving healthy aging and longevity.
2. Biosynthesis of Pas
There are three principal sources of Pas in organisms: dietary intake, cellular synthesis, and gut microbiota synthesis [
12,
13,
14]. Early reports hypothesized that extracellular Pas enter cells through different systems: the heparin sulfate and glypican 1 (GPC1) system, which transports spermine; endocytosis, mediated by Caveolin-1; and the exportation of putrescine by the transporter SLC3A2 [
15]. Recent studies have shown that in different cells and tissues, the transport of spermine and spermidine is executed through polyspecific organic cation transporters 1, 2, and 3 (OTC-1-3) [
16,
17].
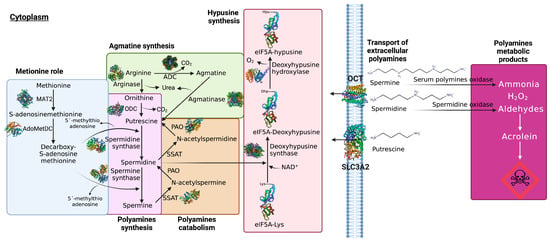
The cellular synthesis of Pas arises from the amino acids arginine, ornithine, and methionine, and the first stage of synthesis includes the production of ornithine and agmatine from arginine catalyzed by arginase (EC 3.5.3.1) and arginine decarboxylase (ADC) (EC 4.1.1.19), respectively. Alternatively, the agmatine could be transformed into putrescine and urea by agmatinase (EC 3.5.3.11). Once ornithine is obtained, it is then decarboxylated by ornithine decarboxylase (ODC) (EC 4.1.1.17) to synthetize putrescine and urea [
18]. ODC is considered the rate-limiting factor of PA synthesis; therefore, its inhibition may be a suitable strategy for the treatment of cancer [
19,
20,
21]. Subsequently, putrescine is converted into spermidine and spermidine is converted into spermine via the action of spermidine synthetase (EC 2.5.1.16) and spermine synthetase (EC 2.5.1.22), respectively [
22] (
Figure 1). Spermidine is also cleaved and transferred by deoxyhypusine synthase (DHS) (EC 2.5.1.46) to eukaryotic translation factor 5A (eIF5A)-Lys to catalyze the interconversion of deoxyhypusine, which is subsequently hydroxylated by deoxyhypusine hydroxylase (DOHH) (EC 1.14.99.29) to produce hypusine. This is considered an unusual amino acid that is essential to the function of eIF5A. The synthesis of hypusine-containing proteins is the most specific post-translational modification known so far, and it is performed enzymatically to complete the maturation of eIF5A [
23,
24] (
Figure 1).
Figure 1. PA transport and metabolism. GPC1 transports spermine, putrescine is exported by the transporter SLC3A2, and spermine and spermidine are transported through OCT–1–3. In the first stage of PA synthesis, ornithine and agmatine are synthetized from arginine catalyzed by arginase and ADC, respectively. Agmatine can be transformed into putrescine and urea by agmatinase. Ornithine is then decarboxylated by ODC to synthetize putrescine and urea. Subsequently, putrescine is converted into spermidine and spermidine is converted into spermine by the action of spermidine synthetase and spermine synthetase, respectively. Spermidine is cleaved and transferred by DHS to eIF5A-Lys to catalyze the interconversion of deoxyhypusine, which is subsequently hydroxylated by DOHH to produce hypusine. In a second stage, dcAdoMet suffers a decarboxylation catalyzed by S–adenosine methionine decarboxylase (pyruvoyl), producing S–adenosine methionine–3–aminopropyl methyl sulfonate. The catabolism pathway of PA successively converts spermine into spermidine and spermidine into putrescine through the acetylated forms of PA (N–acetylspermine and N–acetylspermidine) catalyzed by SSAT and PAO. The degradation of PA leads to the production of highly toxic intermediaries such as aldehydes, peroxides, and ammonia. In particular, aldehydes are extremely reactive and degrade spontaneously, producing acrolein, a highly toxic compound.
In a second stage, S-adenosylmethionine (dcAdoMet) suffers decarboxylation, catalyzed by S-adenosine methionine decarboxylase (pyruvoyl) (EC 4.1.1.50), producing S-adenosine methionine-3-aminopropyl methyl sulfonate (also known as decarboxylated S-adenosyl-l-methionine), which is an aminopropyl donor used by spermidine synthetase and spermine synthetase to produce spermine and spermidine, respectively. Furthermore, the residual 5ߣ-methylthioadenosin (MTA) can induce apoptosis by itself in abnormal cells [
4,
22] (
Figure 1). The catabolism pathway of PA successively converts spermine into spermidine and spermidine into putrescine through the acetylated forms of PA (N-acetylspermine and N-acetylspermidine) catalyzed by spermine/spermidine N1-acetyltransferase (SSAT) (EC 2.3.1.57) and polyamine oxidase (PAO) (EC 1.5.3.11) [
25,
26]. Despite these various physiological functions and cellular needs, Pas are toxic to cells, either due to their high concentrations or via their degradation, which leads to the production of highly toxic intermediaries such as aldehydes, peroxides, and ammonia. Notably, aldehydes are extremely reactive and degrade spontaneously, producing acrolein, a highly toxic compound [
27] (
Figure 1).
3. Polyamines as Novel Biomarkers for Age-Related Diseases
Aging is a natural process; it controls numerous biological and genetic events that are the driving force for all age-related diseases. Although most of the existing research has focused on understanding the molecular mechanisms of aging, developing new strategies for the early diagnosis of age-related diseases should be considered a priority to facilitate better interventions. Our review aims to highlight the advantages of using metabolomics as a risk stratification tool for the detection of age-related diseases. The potential benefits of using metabolomics include a simple approach to new biomarkers in biological fluids or tissues and the provision of predictive information for various clinical variables in age-related diseases [
28,
29].
Because of the plethora of functions of metabolites such as Pas, they can be considered disease indicators. For example, in cancer, they have been widely used in diagnosis and as markers of tumor progression, as cancer patients have elevated levels of polyamines [
30,
31,
32,
33,
34]. As such, there is more than one reason to consider polyamines as novel biomarkers that could indicate the progression of age-related diseases. For example, the most recent work concerning Pas as potential biomarkers in age-related diseases was performed via a metabolomic analysis using serum from individuals with mild cognitive impairment (MCI) and Alzheimerߣs disease, where differentially disrupted levels of polyamines and their metabolites were evident two years before MCI would be diagnosed as AD, demonstrating their high predictive capacity in the progression to AD, although it is necessary to establish specific conditions that can be reproduced worldwide [
35]. Another work group used enzyme-linked immunosorbent assays, showing elevated serum spermidine levels in MCI subjects with underlying AD, again indicating the potential of polyamines as biomarkers for the progression from MCI to AD [
36]. For Parkinsonߣs disease, a metabolomic analysis of the plasma of individuals with the disease showed that polyamine-acetylated metabolites, such as N8-acetylspermidine and N-acetylputrescine, were elevated in PD compared to controls, strongly suggesting that Pas function as useful biomarkers for diagnosis and determining the severity of the disease [
37].
There are very few biomarkers corresponding to age-related diseases linked to mobility disabilities, such as osteoarthritis or sarcopenia, and more are needed. A recent study used a metabolomic analysis to detect the systemic changes in the amino acids and polyamines of individuals with severe OA compared to controls after adjusting the bone mass index (BMI); higher levels of spermidine were found, along with a lower ratio of spermine and spermidine, suggesting their potential use in clinical practice [
38,
39]. The possibility of using polyamines as biomarkers for sarcopenia is almost a reality; the latest research suggests a progressive decrease in the spermine/spermidine ratio in serum from healthy to sarcopenic subjects [
40,
41]. The use of metabolomics is emerging in the osteoporosis field [
42,
43], where the newest evidence highlights the use of the metabolome and its association with fragility fractures; the results have shown higher baseline spermidine levels to be associated with a higher risk of osteoporotic fractures in the Korean community, suggesting a new prognostic biomarker for osteoporosis [
44].
In addition to determining the potential role of PAs as novel biomarkers for age-related diseases, it is crucial to establish large-scale biological and statistical validation for clinical practice. This validation should be conducted across a diverse range of cohorts, including multicentric and unselected prospective cohorts of healthy individuals, as well as clinic-based cohorts. The goal is to ascertain whether monitoring fluctuations in PAs, either independently or in combination with other tools and methods, can improve the precision of preventing and diagnosing disease, assessing disease severity, and tracking the progress of age-related diseases.
4. Polyamines and Neurodegenerative Diseases
It is known that PAs play essential roles in the central nervous system (CNS). PAs are considered primordial stress inducers because they elicit the polyamine stress response (PSR) in response to various temporary stimuli, such as reactive oxygen species (ROS), heat, ultraviolet light (UV), and even aging. This response offers beneficial effects for survival, but its persistent effect leads to other disorders that contribute to neurodegeneration, commonly with arginine deprivation and increased polyamine levels [
61,
62], suggesting that PA levels are important for the replication and maintenance of neurons [
22]. In the mammalian CNS, PAs play a regulatory role in glutamate receptors and have the potential to modulate N-methyl-D-aspartate (NMDA) receptors. These NMDA receptors are crucial for controlling synaptic plasticity, which, in turn, regulates various neurological functions, including memory [
63].
In general, PAs can enhance the opening of channels and regulate glutamate signaling, with an impact on neuronal excitability, memory, and aging [
64]. Aging is the principal risk factor for neurogenerative diseases [
65], which are believed to share the familiar mechanism of the protein aggregation of diverse misfolded proteins, leading to the degeneration of the CNS. The early detection of neurodegenerative diseases could offer an opportunity for the treatment and prevention of disease progression [
66]. In this context, although PAs and their metabolite products have been widely studied in other diseases, there is still the need to understand the connection between the molecular mechanisms of PA metabolism and neurodegenerative diseases in order to identify possible therapeutic approaches for these conditions.
4.1. The Role of PAs in Alzheimer’s Disease
It has long been known that the dysregulation of PA metabolism or PAs’ upstream regulators is involved in the neurodegeneration of the CNS [
37,
45,
67]. Metabolic profiles of the brain have shown that PAs have the capacity to bind the amyloid (A) beta peptide (1–40) to promote the classic aggregation of AD, a complex neurodegenerative disease distinguished by progressive memory and cognitive decline, accompanied by alterations in behavior and visuospatial orientation. AD is the most common cause of dementia [
68]. As mentioned above, in AD, PA levels are disrupted, even though spermidine and spermine significantly increase their levels; however, the possible mechanism has not been completely elucidated [
45,
47,
49]. Nevertheless, the evidence demonstrates that the disruption of arginase, an upstream PA pathway regulator, may constitute one of the main causes of AD-related polyamine metabolism; arginase activity is elevated and, as a result, the deficiency of its substrate, arginine, promotes oxidative stress. Moreover, this facilitates the consumption of ornithine to produce putrescine, which further increases PA levels and the PSR to initiate a cycle of neurodegeneration [
62].
The most recent research about spermidiIe in AD demonstrates two different perspectives on the involvement of spermidine in mild cognitive impairment (MCI) and AD. On the one hand, one research group demonstrated that in 43 samples from American individuals over 65 years old at the Oregon Alzheimer’s Disease Center, an increase in serum levels of spermidine corresponded to the progression from MCI to AD, suggesting the viability of measuring serum spermidine as a key molecule in AD pathology and as a potential biomarker for AD progression [
36]. On the other hand, a study with a rural Chinese population of approximately 3,700 individuals over 35 years old with no history of dementia revealed a non-linear relationship between spermidine and MCI, implying that high levels of spermidine may decrease the burden of MCI [
69]. These contradictory reports raise the possibility that factors such as race, diet, and geographic location could change the general perspective on the role of spermidine in AD or MCI.
In recent years, alterations in autophagic flux have been considered a significant factor in the pathology and progression of AD. Spermidine has been found to induce autophagy by inhibiting the negative regulator EP300 (E1A-binding protein p300) [
70]. This is particularly important due to EP300’s involvement in aging and age-related diseases, including neurodegeneration in AD. Enhancing autophagy, which aids in the removal of accumulated molecules, may offer protection against the disease or potentially delay the onset of the disease [
71,
72]. In addition, novel findings in an AD-like mouse model revealed spermidine’s potential to induce autophagy, showing that spermidine promotes the autophagic degradation of the NLRP3 inflammasome, an essential component of the activation of inflammatory signaling pathways; in this scenario, spermidine represents a promising approach to reducing neuroinflammation in an AD mouse model. Moreover, in the same animal model, spermidine demonstrated an increase in the degradation of soluble amyloid beta peptides, but the condition of the plaques and their size were not altered. These results may lead us to debate the effect of spermidine on AD pathology in comparison with its effect on insoluble amyloid beta peptides [
73].
A polyamine called spermiIwhich is derived from spermidine, has been identified as a molecule that plays a role in the aggregation of Tau protein. This discovery was made via sophisticated experiments using molecular dynamics simulations. These experiments also showed that spermine has a greater affinity for the phosphorylated form of Tau, which, in turn, alters the structure and distribution of Tau and contributes to the formation of fibrillar deposits in neurons [
48]; these findings provide a new outlook for therapeutic development and for understanding the molecular basis of the disease. Further research on PA could lead to the development of interesting therapeutic strategies for neurodegenerative diseases, such as AD. This potential is derived from their natural presence in the human body and the possibility that they could be more effectively tolerated when administered through dietary supplements, either on their own or in combination with other medications.
4.2. The Role of PAs in Parkinson’s Disease
The second most common neurodegenerative disorder is Parkinsonߣs disease (PD), which affects more than 1% of the world’s population over 65 years old; estimation studies indicate its prevalence will double by 2030 [
74]. This disease leads to alterations in cardinal motor features, slowed movement, rigidity, and tremors, along with other non-motor disturbances such as cognitive decline; these represent the heterogeneity of the symptom burden. As is the case in AD, protein aggregation is a molecular hallmark of PD. Alpha-synuclein (α-synuclein) accumulates in intraneuronal inclusions, causing toxicity and cellular dysfunction [
74].
More than a decade ago, it was demonstrated that PD patients with a worse phenotype of the disease showed an increase in putrescine levels in their cerebrospinal fluid (CSF), along with a decrease in spermidine levels [
54]. To date, the molecular role and regulation of the PA levels in a healthy brain or a brain with PD are not well elucidated. Even less is known about how a polyamine’s metabolic changes might impact neurodegenerative diseases. However, a recent study shed light on certain forms of acetylated polyamines, particularly spermidine, which were significantly elevated in the blood serum of PD patients when compared to control groups. Spermidine, a product of the interconversion between spermidine and spermine catalyzed by Spd/Spm acetyltransferase, is now being proposed as a potential biomarker for diagnosing PD and assessing its severity. These findings could also help distinguish PD from other neurological diseases such as AD and progressive supranuclear palsy (PSP) [
37].
Regarding protein homeostasis in PD, one interesting molecule is ATP13A2, a lysosomal transporter with five different transmembrane domains. ATP13A2 plays an essential role in maintaining neuronal wellbeing through the regulation of metal ions and the organelle homeostasis of the endoplasmic reticulum, lysosomes, and mitochondria. Interestingly, ATP13A2 also promotes the degradation of polyamines and α-synuclein [
75]. The loss of ATP13A2 has been reported in an atypical form of PD, which was determined to cause dysfunction in lysosomal membrane integrity, as it alters the dysfunction of spermidine/spermine exports and consequently promotes the accumulation of α-synuclein [
76,
77]. Nowadays, efforts to measure the ATP13A2 levels in serum or saliva raise the possibility of it functioning as a potential marker of PD development and complications [
78,
79]. In general, these findings highlight the importance of studying PA export dysfunction related to ATP13A2 loss in neurodegeneration and through autophagy regulation, which may represent a therapeutic target for delaying these neurodegenerative conditions.