Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Visceral sarcomas are a rare malignant subgroup of soft tissue sarcomas (STSs). STSs, accounting for 1% of all adult tumors, are derived from mesenchymal tissues and exhibit a wide heterogeneity. Their rarity and the high number of histotypes hinder the understanding of tumor development mechanisms and negatively influence clinical outcomes and treatment approaches.
- preclinical models
- cancer-derived cells
- patient-derived xenografts
1. Cell-Derived and Patient-Derived Xenograft Models of Soft Tissue Sarcomas
Among the available animal models in cancer research, human tumor xenografts are the most widely used. They consist of the implantation of tumor cells into immunocompromised mice, either under the skin or into the same organ type of origin (Figure 1A). Athymic nude mice, severe combined immunodeficient (SCID) mice, or other immunocompromised mice can readily accept xenografts [1].
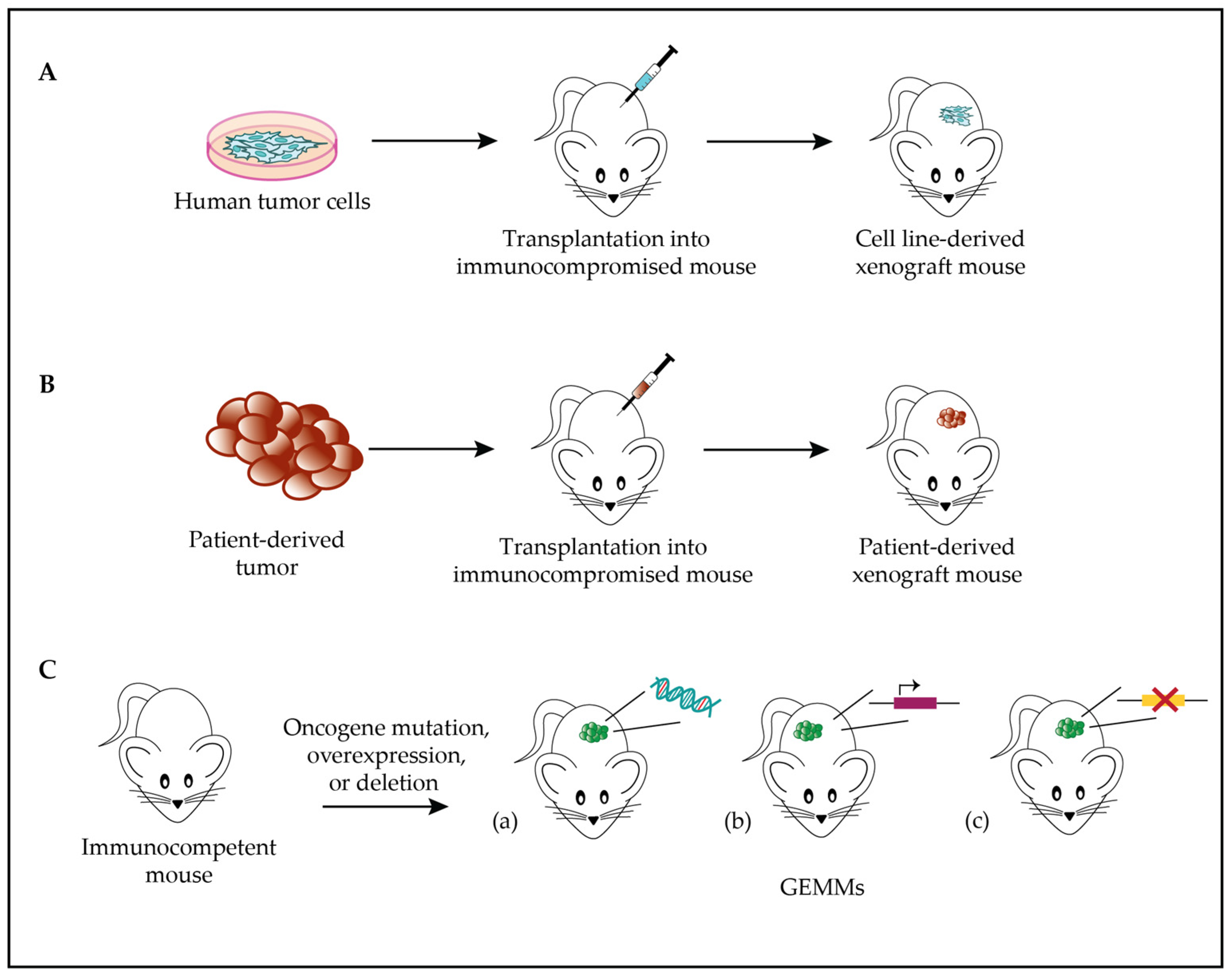
Figure 1. Soft tissue sarcomas (STS) models. (A). Scheme of cell line-derived xenograft model generation: tumor cells are transplanted into immunocompromised mice to study tumor biology and behavior. (B). Scheme of PDX model generation: patient-derived tumor cells are transplanted into immunocompromised mice to study tumor biology and behavior. (C). Genome-engineered mouse model: GEMMs drive tumor development via mutation (a), overexpression (b), or deletion (c) of one or several oncogenes.
Several studies have been conducted on cell-derived xenograft (CDX) models to assess both the target therapy effectiveness and oncogenic activity in STSs. An example is the xenograft model investigated by Floris and colleagues, obtained from the IM-sensitive GIST882 cell line. For the first time, they used this nude xenograft model to evaluate the effects of the HDACi panobinostat on human GISTs with different oncogenic KIT mutations. Panobinostat reduced proliferation and increased apoptosis in all xenografts, proving its anti-tumor activity [2]. Another STS xenograft model, applied to leiomyosarcomas, is the one established by Zhang‘s group by injecting SCID mice with SK-LMS-1 cells previously transfected with VEGF165 [3]. Approximately 21–25% of patients affected by STSs display VEGF overexpression, which correlates with a more advanced tumor grade and a worse prognosis [4][5]. Based on the established role of VEGF and its receptor VEGFR in angiogenesis, the authors tried to better clarify the role of VEGF165 in STS growth, metastasis, and chemoresistance. The obtained VEGR165-overexpressing xenograft model revealed the significant impact of VEGF expression on STSs’ ability to grow and metastasize, and the anti-VEGFR2 monoclonal antibody’s effects on enhancing the doxorubicin response [3]. CDX models provide the advantage of replicating human tumor biology, despite the possibility of clone selection, which might not accurately represent the human disease [6].
An alternative to cell line xenografts is the transplantation of small pieces of human-derived tumor samples into mice, obtaining so-called patient-derived xenograft (PDX) models (Figure 1B). In this case, the advantage is represented by the close resemblance of the model to the primary tumor sample [7]. For instance, copy number alterations observed in STS PDXs are also detected in sarcoma patients, suggesting that these alterations correlate with actual tumor progression rather than experimental model alterations [8]. Several models have been successfully established, achieving an overall engraftment success rate of 32% to 69% and efficiently reproducing the genetic and phenotypic characteristics of the original tumor [9][10].
PDXs can be considered superior to classical cell-line-derived xenografts in accurately predicting patient response to therapy [11][12]. In this regard, Gebreyohannes and colleagues proved the anti-tumor efficacy of cabozantinib, a novel tyrosine kinase inhibitor, by reducing tumor growth, proliferation, and angiogenesis in IM-sensitive and IM-resistant PDX models of GISTs [13]. Similarly, Van Looy’s team demonstrated the effectiveness of three PI3K inhibitors combined with imatinib in reducing tumor volumes and enhancing apoptosis in GIST PDXs [14]. Analogous research has been conducted on sarcoma xenograft models to explore the antitumor effects of target therapies. This is the case for Li and colleagues, who established two PDX models of DDLPS by implanting pieces of patient-derived sarcomas inro athymic nude NMRI mice. Significantly decreased proliferation and angiogenesis inhibition characterized all the xenografts treated with the TKRi pazopanib [15]. Zuco’s group conducted a direct comparison between the first-in-class XPO1 inhibitor selinexor and doxorubicin, the standard front-line therapy for sarcomas, in three DDLPS PDXs. They demonstrated selinexor’s potential as a therapeutic agent for DDLPS, given its superiority in terms of the umor response in all PDXs when compared to doxorubicin, regardless of the MDM2 amplification and histological differentiations [16].
The few preclinical models of leiomyosarcoma and the lack of fidelity of the established LMS cell lines to their mesenchymal neoplasm of origin limit the translational understanding of the disease. In this regard, Hemming and colleagues characterized LMS PDX models assessing that, across multiple xenograft passages, the parental tumor histological appearance, copy number variation, and transcriptional program were maintained. Additionally, LMS PDXs were susceptible to cyclin-dependent kinase (CDK) inhibition, which alters the oncogenic transcriptional program driven by E2F and hinders tumor growth. Thus, CDK inhibitors can be a valuable therapeutic strategy for patients with LMS [17].
As previously discussed, PDXs are useful to assess the effects of combined therapies. This was further supported by Perez’s team, who demonstrated the potential therapeutic strategies of doxorubicin and olaparib against sarcomas in UPS PDXs. The combined treatment efficacy in tumors with high levels of pH2AX and MAP17 suggested that both biomarkers could potentially identify patients who would benefit more from the therapy [18]. Similarly, Stacchioti’s group investigated the preclinical antitumor activity of EPZ-011989, an EZH2 inhibitor, in INI1-deficient proximal-type epithelial sarcoma (ES) PDXs. EPZ-011989, gemcitabine, and a doxorubicin–ifosfamide combination exhibited comparable antitumor activity in treated mice, supporting their clinical use as effective therapies. Moreover, EZH2 was confirmed as a viable therapeutic target in ESs, suggesting autophagy as a possible protective mechanism against EZH2 inhibition [19].
Interestingly, an established patient-derived orthotopic nude mouse xenograft of a retroperitoneal STS was developed by Hiroshima and colleagues. This model recapitulated the histology of the original tumor better than the same subcutaneous ectopic model [20]. Hence, PDXs are largely used for the assessment of human tumor biology and have a broad range of applications in preclinical drug testing, in therapeutic target identification, and for the establishment of stable xenograft cell lines [6][21]. Furthermore, these models could represent an option for personalized medicine strategies, allowing for direct testing of potential drug treatments on a model matching the patient [6]. However, recent advances in immunotherapy highlighted the importance of immune response in cancer progression and treatment, and thus the need to develop new PDX models to investigate human cancer and immune system interactions [21]. Wang’s team established an in vivo humanized mouse xenograft model by transplanting human CD34+ hematopoietic progenitor and stem cells into NGS mice, originating humanized NGS (HuNGS) mice with human hematopoietic and immune systems. Then, they implanted PDXs of various cancers (sarcoma, non-small cell lung cancer, bladder, triple-negative breast cancer) into HuNGSs. Treatment with PD-1 checkpoint inhibitor pemrolizumab significantly inhibited the tumor growth of PDX tumors in HuNGS mice, assessing the potential utility of the model for preclinical immunotherapy research [22].
Patient-derived models (PDMs) are widely applied in cancer research, drug development, and clinical applications. Since PDMs are directly derived from patients, they can predict treatment response and could help to identify the best personalized treatment strategy [11][23]. Research findings support that sarcoma patient-derived cells predict STS patient response to therapy since these models preserve the genetic characteristics of the original tumor and the association between drug sensitivity and patient response [24]. Nonetheless, there are still challenges and limitations to overcome, such as costs and time, as well as tumor heterogeneity, which might not be represented by PDMs, potentially affecting the accuracy of drug testing [11][23].
2. Genome-Engineered Mouse Models of Soft Tissue Sarcomas
Among other animal models used in sarcoma research, it is worthwhile to mention the genome-engineered mouse model (GEMM), in which mice display an altered genetic profile by mutating, deleting, or overexpressing one or several oncogenes (Figure 1C). GEMMs allow researchers to monitor the effects of induced genetic alterations over time and to evaluate tumor response to treatment in vivo [25]. Conditional transgenic mice expressing oncogenic human fusion genes, as well as immunodeficient mice that enable the growth of human tumor cells or tumor fragments cultured in vitro, have allowed the implementation of the available preclinical models for translational research [26].
An example of an in vivo visceral sarcoma model is represented by the MMTV-CR-1 transgenic mice generated by Strizzi and colleagues, in which CR-1 transgene expression is regulated by the MMTV (the mouse mammary tumor virus) long terminal repeat promoter and leads to uterine leiomyosarcoma development. CR-1 plays a role in uterine tumor onset via the direct activation of c-src and Akt or via crosstalk with the canonical Wnt signaling pathway [27].
Many transgenic mouse models have also been generated to study gastrointestinal stromal tumors. For instance, Sommer’s group created a knock-in mouse with an exon 11 KIT-activating mutation (KITV558 deletion), previously found in the case of human familial GIST syndrome. Through this model, they reproduced gastrointestinal pathology in mice with remarkable penetrance, demonstrating that the constitutive activation of KIT signaling is pivotal and sufficient to cause neoplastic growth in mice [28]. Likewise, Rubin’s team developed a homozygous knock-in mouse model harboring a KIT-activating mutation K641E, resulting in GIST development with 100% penetrance. They also showed the model effectiveness for the study of KIT pathway activation, GIST pathogenesis, and preclinical validation of GIST therapies and drug response [29].
Regarding the study of undifferentiated pleomorphic sarcoma, for the first time, Buchakjian and colleagues generated a viral Cre-mediated TRP53/PTEN mouse model, by injecting adenoviral Cre recombinase into TRP53flox/flox/PTENflox/flox lox–stop–lox luciferase mice. All the injected mice developed STSs, identified for 93% as invasive pleomorphic sarcomas characterized by lymphocytic infiltrate (64%) and PD-L1 expression (71%). The model could represent a valuable tool for liposarcoma preclinical studies since the homozygous loss of TRP53 and PTEN in mouse adipose tissue also characterizes this sarcoma histotype [30].
Within the realm of liposarcoma research, Pèrez-Mancera’s group generated CHOP and FUS ± CHOP transgenic mice, by introducing CHOP or the FUS-CHOP transgene into mouse genomes. Interestingly, only the latter mouse group developed LPSs. This demonstrated the critical role of the FUS domain in the liposarcoma pathogenesis and pioneeringly proved the in vivo correlation between gene fusions and solid tumor onset [31]. A few years later, the same group generated double-transgenic FUS-CHOP mice to investigate the significance of the FUS-CHOP interaction. As a result, FUS expression in CHOP transgenic mice restored liposarcoma development, indicating that the FUS and CHOP domains cooperate in mutual liposarcoma restoration [32].
As previously mentioned, unfortunately, angiosarcomas lack valid clinical models that allow new therapy development. However, Salter’s team generated an autochthonous AS mouse model driven by p53 deregulation in VE-cadherin-expressing endothelial cells, using Cdh5-Cre mice. AS arose in mice with a penetrance of 100% upon homozygous deletion of TRP53. The re-implantation of AS fragments from Cdh5-Cre, Trp53fl/fl mice yielded a reliable and rapid angiosarcoma model. Moreover, transferring tumor fragments within mice allowed them to establish a novel AS model suitable for preclinical studies and for new therapy development [33].
This entry is adapted from the peer-reviewed paper 10.3390/biom13111624
References
- Morton, C.L.; Houghton, P.J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2007, 2, 247–250.
- Floris, G.; Debiec-Rychter, M.; Sciot, R.; Stefan, C.; Fieuws, S.; Machiels, K.; Atadja, P.; Wozniak, A.; Faa, G.; Schöffski, P. High efficacy of panobinostat towards human gastrointestinal stromal tumors in a xenograft mouse model. Clin. Cancer Res. 2009, 15, 4066–4076.
- Zhang, L.; Hannay, J.A.F.; Liu, J.; Das, P.; Zhan, M.; Nguyen, T.; Hicklin, D.J.; Yu, D.; Pollock, R.E.; Lev, D. Vascular endothelial growth factor overexpression by soft tissue sarcoma cells: Implications for tumor growth, metastasis, and chemoresistance. Cancer Res. 2006, 66, 8770–8778.
- Potti, A.; Moazzam, N.; Langness, E.; Sholes, K.; Tendulkar, K.; Koch, M.; Kargas, S. Immunohistochemical determination of HER-2/neu, c-Kit (CD117), and vascular endothelial growth factor (VEGF) overexpression in malignant melanoma. J. Cancer Res. Clin. Oncol. 2004, 130, 80–86.
- Potti, A.; Ganti, A.K.; Tendulkar, K.; Sholes, K.; Chitajallu, S.; Koch, M.; Kargas, S. Determination of vascular endothelial growth factor (VEGF) overexpression in soft tissue sarcomas and the role of overexpression in leiomyosarcoma. J. Cancer Res. Clin. Oncol. 2004, 130, 52–56.
- Hamacher, R.; Bauer, S. Preclinical models for translational sarcoma research. Curr. Opin. Oncol. 2017, 29, 275–285.
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350.
- Kresse, S.H.; Meza-Zepeda, L.A.; Machado, I.; Llombart-Bosch, A.; Myklebost, O. Preclinical xenograft models of human sarcoma show nonrandom loss of aberrations. Cancer 2012, 118, 558–570.
- Cornillie, J.; Wozniak, A.; Li, H.; Wang, Y.; Boeckx, B.; Gebreyohannes, Y.K.; Wellens, J.; Vanleeuw, U.; Hompes, D.; Stas, M.; et al. Establishment and Characterization of Histologically and Molecularly Stable Soft-tissue Sarcoma Xenograft Models for Biological Studies and Preclinical Drug Testing. Mol. Cancer Ther. 2019, 18, 1168–1178.
- Stewart, E.; Federico, S.M.; Chen, X.; Shelat, A.A.; Bradley, C.; Gordon, B.; Karlstrom, A.; Twarog, N.R.; Clay, M.R.; Bahrami, A.; et al. Orthotopic Patient-Derived Xenografts of Pediatric Solid Tumors. Nature 2017, 549, 96–100.
- Pompili, L.; Porru, M.; Caruso, C.; Biroccio, A.; Leonetti, C. Patient-derived xenografts: A relevant preclinical model for drug development. J. Exp. Clin. Cancer Res. 2016, 35, 189.
- Imle, R.; Kommoss, F.K.F.; Banito, A. Preclinical in vivo modeling of pediatric sarcoma—Promises and limitations. J. Clin. Med. 2021, 10, 1578.
- Gebreyohannes, Y.K.; Schöffski, P.; Van Looy, T.; Wellens, J.; Vreys, L.; Cornillie, J.; Vanleeuw, U.; Aftab, D.T.; Debiec-Rychter, M.; Sciot, R.; et al. Cabozantinib is active against human gastrointestinal stromal tumor xenografts carrying different KIT mutations. Mol. Cancer Ther. 2016, 15, 2845–2852.
- Van Looy, T.; Wozniak, A.; Floris, G.; Sciot, R.; Li, H.; Wellens, J.; Vanleeuw, U.; Fletcher, J.A.; Manley, P.W.; Debiec-Rychter, M.; et al. Phosphoinositide 3-kinase inhibitors combined with imatinib in patient-derived xenograft models of gastrointestinal stromal tumors: Rationale and efficacy. Clin. Cancer Res. 2014, 20, 6071–6082.
- Li, H.; Wozniak, A.; Sciot, R.; Cornillie, J.; Wellens, J.; Van Looy, T.; Vanleeuw, U.; Stas, M.; Hompes, D.; Debiec-Rychter, M.; et al. Pazopanib, a receptor tyrosine kinase inhibitor, suppresses tumor growth through angiogenesis in dedifferentiated liposarcoma xenograft models. Transl. Oncol. 2014, 7, 665–671.
- Zuco, V.; Pasquali, S.; Tortoreto, M.; Brich, S.; Percio, S.; Dagrada, G.P.; Colombo, C.; Sanfilippo, R.; Lauricella, C.; Gounder, M.; et al. Selinexor versus doxorubicin in dedifferentiated liposarcoma PDXs: Evidence of greater activity and apoptotic response dependent on p53 nuclear accumulation and survivin down-regulation. J. Exp. Clin. Cancer Res. 2021, 40, 83.
- Hemming, M.L.; Bhola, P.; Loycano, M.A.; Anderson, J.A.; Taddei, M.L.; Doyle, L.A.; Lavrova, E.; Andersen, J.L.; Klega, K.S.; Benson, M.R.; et al. Preclinical Modeling of Leiomyosarcoma Identifies Susceptibility to Transcriptional CDK Inhibitors through Antagonism of E2F-Driven Oncogenic Gene Expression. Clin. Cancer Res. 2022, 28, 2397–2408.
- Perez, M.; García-Heredia, J.M.; Felipe-Abrio, B.; Muñoz-Galván, S.; Martín-Broto, J.; Carnero, A. Sarcoma stratification by combined pH2AX and MAP17 (PDZK1IP1) levels for a better outcome on doxorubicin plus olaparib treatment. Signal Transduct. Target. Ther. 2020, 5, 195.
- Stacchiotti, S.; Zuco, V.; Tortoreto, M.; Cominetti, D.; Frezza, A.M.; Percio, S.; Indio, V.; Barisella, M.; Monti, V.; Brich, S.; et al. Cancers Comparative Assessment of Antitumor Effects and Autophagy Induction as a Resistance Mechanism by Epithelioid Sarcoma Patient-Derived Xenograft. Cancers 2019, 11, 1015.
- Hiroshima, Y.; Zhang, Y.; Zhang, N.; Uehara, F.; Maawy, A.; Murakami, T.; Mii, S.; Yamamoto, M.; Miwa, S.; Yano, S.; et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer. Res. 2015, 35, 697–701.
- Tratar, U.L.; Horvat, S.; Cemazar, M. Transgenic mouse models in cancer research. Front. Oncol. 2018, 8, 268.
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549.
- Sabaawy, H.E.; Broggini, M.; Gupta, S.K. Editorial: Patient-derived tumor models for drug development. Front. Oncol. 2023, 13, 1243534.
- Brodin, B.A.; Wennerberg, K.; Lidbrink, E.; Brosjö, O.; Potdar, S.; Wilson, J.N.; Ma, L.; Moens, L.N.; Hesla, A.; Porovic, E.; et al. Drug sensitivity testing on patient-derived sarcoma cells predicts patient response to treatment and identi fi es c-Sarc inhibitors as active drugs for translocation sarcomas. Br. J. Cancer 2019, 120, 435–443.
- Richmond, A.; Yingjun, S. Mouse xenograft models vs GEM models for human cancer therapeutics. DMM Dis. Model. Mech. 2008, 1, 78–82.
- Landuzzi, L.; Ruzzi, F.; Lollini, P.L.; Scotlandi, K. Synovial Sarcoma Preclinical Modeling: Integrating Transgenic Mouse Models and Patient-Derived Models for Translational Research. Cancers 2023, 15, 588.
- Strizzi, L.; Bianco, C.; Hirota, M.; Watanabe, K.; Mancino, M.; Hamada, S.; Raafat, A.; Lawson, S.; Ebert, A.D.; D’Antonio, A.; et al. Development of leiomyosarcoma of the uterus in MMTV-CR-1 transgenic mice. J. Pathol. 2007, 211, 36–44.
- Sommer, G.; Agosti, V.; Ehlers, I.; Rossi, F.; Corbacioglu, S.; Farkas, J.; Moore, M.; Manova, K.; Antonescu, C.R.; Besmer, P. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 6706–6711.
- Rubin, B.P.; Antonescu, C.R.; Scott-Browne, J.P.; Comstock, M.L.; Gu, Y.; Tanas, M.R.; Ware, C.B.; Woodell, J. A knock-in mouse model of gastrointestinal stromal tumor harboring Kit K641E. Cancer Res. 2005, 65, 6631–6639.
- Buchakjian, M.R.; Merritt, N.M.; Moose, D.L.; Dupuy, A.J.; Tanas, M.R.; Henry, M.D. A Trp53fl/flPtenfl/fl mouse model of undifferentiated pleomorphic sarcoma mediated by adeno-Cre injection and in vivo bioluminescence imaging. PLoS ONE 2017, 12, e0183469.
- Pérez-Losada, J.; Pintado, B.; Gutiérrez-Adán, A.; Flores, T.; Bañares-González, B.; Del Campo, J.C.; Martín-Martín, J.F.; Battaner, E.; Sánchez-García, I. The chimeric FUS/TLS-CHOP fusion protein specifically induces liposarcomas in transgenic mice. Oncogene 2000, 19, 2413–2422.
- Pérez-Mancera, P.A.; Pérez-Losada, J.; Sánchez-Martín, M.; Rodríguez-García, M.A.; Flores, T.; Battaner, E.; Gutiérrez-Adán, A.; Pintado, B.; Sánchez-García, I. Expression of the FUS domain restores liposarcoma development in CHOP transgenic mice. Oncogene 2002, 21, 1679–1684.
- Salter, D.M.; Griffin, M.; Muir, M.; Teo, K.; Culley, J.; Smith, J.R.; Gomez-Cuadrado, L.; Matchett, K.; Sims, A.H.; Hayward, L.; et al. Development of mouse models of angiosarcoma driven by p53. Dis. Model. Mech. 2019, 12, dmm038612.
This entry is offline, you can click here to edit this entry!
