Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sewage sludge ash (SSA) is a rich source of P, while municipal solid waste incineration fly or bottom ashes (MSWI-FA, MWSI-BA) are rich in metals, especially Zn, Pb, and Cu.
- critical raw materials
- extraction
- sewage sludge ash
- municipal solid waste incineration ash
- wood biomass ash
1. Introduction
The European Commission (EC) has recognized the necessity to recover critical raw materials (CRMs), not only from primary but also from secondary sources as much as possible. In 2015, the document “Closing the loop—An EU action plan for the Circular Economy” expressed the EC’s aim to support the Raw Materials Information System, which would provide data on secondary raw materials (SRMs). SRMs can be sources used instead of primary raw materials. SRMs can also be a source for CRMs, which are of high economic importance due to their expected scarcity in the EU market or a high risk of supply disruption. The EU thus wishes to promote recycling these materials, especially the recovery of CRMs, as part of the move towards the circular economy [1].
One CRM is phosphorus (P), which is an irreplaceable resource and an essential nutrient for the growth of organisms [2,3]. Most P is currently extracted from phosphate rocks, which are the primary and non-renewable sources occurring in a limited number of deposits worldwide [4]. Most of the world’s phosphate rock deposits are located in Morocco, while the rest are found in China, the United States, South Africa, and Jordan [5]. These global P resources will be depleted within a few decades, which is why the EC has added P and phosphate rock to the list of CRMs shown in Figure 1 [6]. Solutions for a P recovery from various secondary resources such as ashes are hence required. Optimized use of P in agriculture and soil stabilization to prevent erosion is additionally encouraged. Rare earth elements (REEs) are another limited resource, and the EC placed them in the CRMs list due to the increasing demand for them in current industrial production (e.g., permanent magnets required for electric motors or wind energy). They are sorted into the groups of light and heavy REEs (LREEs and HREEs, respectively) [6,7].
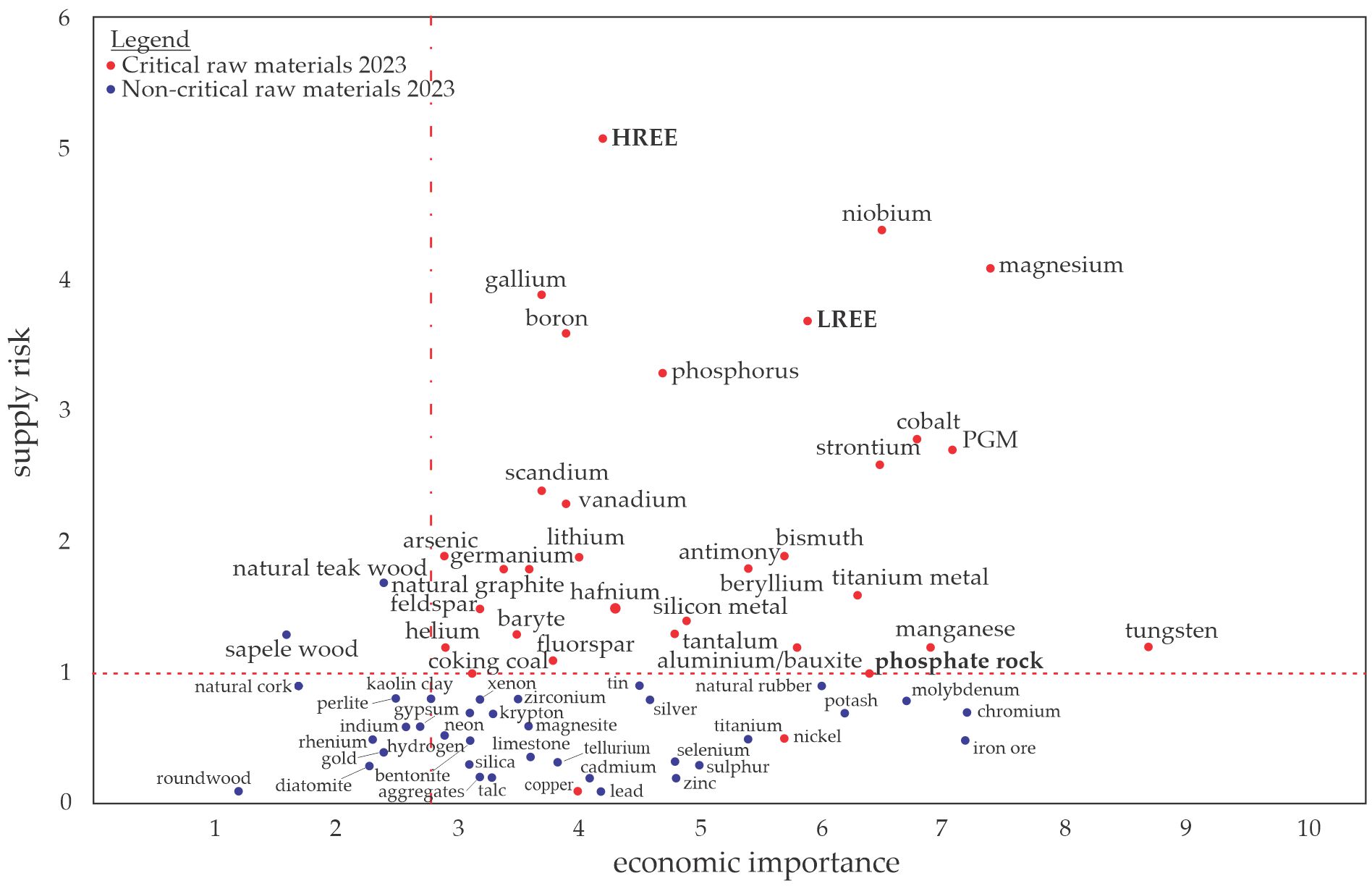
Figure 1. Raw materials (CRMs list from 2023) in relation to their economic importance and supply risk, redrawn based on an EU report [6]. The “critical area” is defined by a supply risk ≥ 1 and an economic importance ≥ 2.8.
Even apart from CRMs, there is a fundamental dependence on metals in different industries. Non-ferrous base metals, such as Cu, Zn, and Pb, are some of the most used metals worldwide, only exceeded by Fe and Al. All these elements are necessary for our current society and infrastructure. Projections predict an increase in the demand for Cu by 140%, Zn by 81%, and Pb by 46% compared to the 2010 demand until 2050 [8]. By the end of the 21st century, the projected demand is even higher for Cu and Zn (330% for Cu and 130% for Zn) [8]. At the same time, the expected depletion years for these elements (the year when the cumulative primary production exceeds the reserves) can be reached before 2025–2038 without the peak year of primary production being reached [8]. Consequently, used ore grades become lower, which typically leads to an increased energy demand for extraction, e.g., for fossil fuels, resulting in increasing greenhouse gas emissions for constant production [9,10]. This calls for developing new economically viable mining methods and extraction strategies applicable to SRMs. The use of elements in products usually dilutes their concentration [11], whereas incinerating end-of-life products causes the accumulation of elements in incineration residues, which can hence be an important source for improving the circularity of elements otherwise deposited in landfills.
Table 1 compares the concentrations of P and selected heavy metals reported in various ashes with typical concentrations in mined ores. Sewage sludge ash (SSA) is a rich source of P, while municipal solid waste incineration fly or bottom ashes (MSWI-FA, MWSI-BA) are rich in metals, especially Zn, Pb, and Cu. Wood biomass fly or bottom ashes (WB-FA, WB-BA), on the other hand, do not have a very high P content but contain essential micronutrients for plants as well as Na [12]. The composition of the biomass varies depending on the combusted biomass type [12,13,14,15]. REEs are generally not enriched in these ashes in comparison to the concentrations found in the Earth’s crust [7]; combined REE concentrations of 88–124 mg/kg were found in MSWI-BA and averaged 54 mg/kg in MSWI-FA sampled in Italy [16]. Biomass ashes contain even lower combined REE concentrations of, e.g., ~27 mg/kg [17]. Recovering REEs from such ashes may still become economical as they can be co-extracted with the other resources in Table 1, and their demand is expected to reach a ~4.4% annual growth rate globally by 2026 [18] and rise by 2600% over the next 25 years [19]. Despite extensive, mostly lab-scale research efforts on REE recycling, less than 1% were recycled in 2011 due to inefficient collection, technological problems, and a lack of incentives [20]. Achieving a circular economy and a truly sustainable society must include the recovery of REEs from SRMs with low concentrations [7].
Table 1. Minimum and maximum contents of P and selected heavy metals in treated SSA, MSWI-FA, MSWI-BA, and wood biomass fly and bottom ashes (WB-FA, WB-BA) compared to typical ore concentrations.
| Element | SSA [2,4,21,22,23,24,25] |
MSWI-FA [15,26,27] |
MSWI-BA [26] |
WB-FA [12,28,29] |
WB-BA [28] |
Typical Ore Concentration [30,31] |
|---|---|---|---|---|---|---|
| P (g/kg) * | 35–99 | 4–5 | - | 10–23 | 8–17 | 110–160 |
| Zn (mg/kg) | 895–2823 | 9000–70,000 | 610–7800 | 446–2274 | 74–234 | 50,000–150,000 |
| Pb (mg/kg) | 70–460 | 5300–26,000 | 100–13,700 | 11–177 | 5–80 | 300,000–400,000 |
| Cu (mg/kg) | 423–839 | 600–3200 | 190–8200 | 89–161 | 65–111 | 5000–20,000 |
| Cr (mg/kg) | 78–460 | 140–1100 | 23–3200 | 18–101 | 25–70 | 310,000 |
| Cd (mg/kg) | 4–126 | 50–450 | 0.3–70 | 7–16 | 0.1–0.5 | 1000–10,000 |
* Calculated based on the P2O5 content.
2. Recovery of Resources from Selected Ashes
2.1. Overview of Extraction Technologies
Various techniques, such as wet extraction, thermochemical, and electrochemical methods, have been developed to extract or recover metals from different ashes [35,36]. This overview also covers processes at the technology readiness level (TRL) 7 (see Section 2.2) using wet extraction methods. Wet chemical extraction is the most widely used method for extracting P from various ashes due to its high recovery rate, low cost, and procedure simplicity. Choosing the right extractant is very important. Common examples are inorganic acids such as sulfuric acid (H2SO4) [2,4,22,23,24,25,37,38,39], nitric acid (HNO3) [4,23,25,37,39], and hydrochloric acid (HCl) [4,15,40,41]; organic acids and chelating agents such as citric acid (CA) [25,37,38,39], oxalic acid (OA) [4,22,25,37,38,39], lactic acid (LA) [37], ethylenediaminetetraacetic acid (EDTA) [25,38,39], and ethylenediamine tetra (methylene phosphonic acid) (EDTMP) [25,38,39]; and bases such as NaOH [4]. Novel chelating agents for ashes could also be bisphosphonates [42].
The most commonly used inorganic acid and the cheapest extractant on today’s market is H2SO4 [23]. Its main advantages are easy transportation due to its low volatility, the possibility of concentrating up to 98%, and ensuring less co-dissolution of heavy metals, especially Pb [2,4]. Other inorganic acids, such as HNO3 [4,23,25,37,39], HCl [4,15,40,41], and H3PO4 [5], have also been used. HCl may facilitate the occurrence of unwanted complexation reactions [2], and H3PO4 is comparably expensive [5]. Organic acids are usually chosen in research for their reduction properties (especially OA, which is the strongest naturally occurring organic acid) and for their environmentally friendly production (CA and LA) [22,25,37,38,39]. OA is the most efficient P extractant among the organic acids as it combines a high P extraction efficiency with a relatively low co-extraction of heavy metals; however, H2SO4 has an economic advantage over OA due to the lower costs for optimal P extraction [4,25].
Chelating agents have a marginal effect on the morphology and particle size distribution of ashes and are less effective for P recovery compared to inorganic and organic acid extractants due to their high affinity to metal ions, resulting in partial dissolution of P [34,39]. EDTA performed better than EDTMP for trace elements such as Zn, Pb, or Cd, so EDTA could be used ahead of the P extraction to remove significant quantities of metals without leaching P [38]. Fang et al. also published a study where a combination of EDTA and H2SO4 was used but was ineffective for P extraction [43]. The use of Cyanex, a highly stable P-based chelating extractant, is effective for the extraction of heavy metals (Zn, Pb, and Cd) from leachates after wet extraction [27,44]. However, the selective separation of REEs requires very specific kinds of chelating agents, where bisphosphonates could be a promising option [42]. They contain a carbon center with two phosphonate groups (PO(OH)2) and two other substituents (e.g., a hydroxyl group and a carbon chain with a primary amine group) [45]. Bisphosphonates have been immobilized on nanoporous silicon and then applied to selectively recover Sc from a highly complex ore sample leaching solution [42]; a similar approach could be applied to ashes. The desorption of Sc from bisphosphonates can be conducted by using acids such as H3PO4 and H2SO4 [42]. Bisphosphonates can also be used for a relatively selective recovery of other REEs, but information remains scarce [46].
Alkali-metal bases such as NaOH dissolve almost no heavy metals, mainly due to the high pH of around 13 at the end of the extraction procedure. They are also ineffective for P extraction because Ca-phosphates show poor solubility in alkaline environments (especially when the molar P/Ca ratio is lower than one), while Al- and Fe-phosphates are highly soluble in such media [4,24].
Optimal process conditions require combining the highest P extraction efficiency, the lowest possible co-extraction of heavy metals, and the lowest possible operating costs. Additionally, variables such as the extractant type and its concentration, the contact time (optimally 2 h [2,22,24,38,39]), the liquid/solid ratio (optimally 20:1 [2,23,24,38]), the extraction temperature and ash composition significantly affect the P extraction efficiency [2,22,24]. Longer extraction times (e.g., one week compared to 2 h) result in a lower P extraction efficiency and more heavy metal leaching [23]. It is also necessary to consider the variability of certain elemental contents, e.g., the P content in SSA can be partially attributed to differences in wastewater treatment systems and incineration conditions [47]. The sampling period also appears to influence its content, as, e.g., a lower P content has been measured in the summer months while higher contents were measured in February and March [48]. This can be explained by the different food habits and leisure activities of people in different seasons [48]. The recovery of P by wet chemical extraction can be effectively applied to different types of SSA, as they contain higher amounts of P [2,4,22,23,24,25,37,38,39]. Ashes from wood biomass and MSWI are not as rich in P as SSA, but they are rich in Zn, which is also important to recover [40,44,49,50,51].
An overview of the extractants used to extract P, Zn, Pb, Cu, Cr, and/or Cd from the selected ashes is presented in Table 2, along with the respective achieved extraction rates. In the case of P, the highest extraction rate of 100% was achieved using H2SO4 [23], HNO3 [23,37], and OA [22], while the most effective extractant for Zn was HCl, reaching 86% [41]. The highest reported extraction rate in the analyzed literature for Cr was 58% [39], 74% for Cu [38], 62% for Pb [40], and 97% for Cd [40]. Although chemical extraction achieves high efficiencies, it requires further purification and the treatment of insoluble acid residues [22,34,38,52,53]. It also often requires undesirably large amounts of acids, encouraging researchers to develop alternative methods [35]. One alternative for achieving high extraction rates of Zn (around 90%), Pb, and Cd from ash is the thermochemical method, but concerns regarding its operating costs, high energy input, and equipment lifetimes have been voiced [52,53,54]. KCl, MgCl2, and CaCl2 are often added in the thermochemical process, where high concentrations of chlorine compounds can be extremely corrosive [53,55,56]. Increasing the treatment temperature led to higher Pb and Zn removal rates [55], and above 1400 °C, the thermal removal of heavy metals also enabled the separation of Fe, increasing the bioavailability of P in the ash [53]. The most promising one-step extraction method is EDS, whose main advantage over other techniques is the ability to separate P from the remaining waste and remove heavy metals from ash in one process [3,57,58,59,60]. Here, electromigration separates the component by transporting P towards the anode and heavy metals mainly towards the cathode, a very important aspect considering mixed component wastes [57]. After the treatment, P is recovered from the anolyte by filtration to separate the liquid from the remaining solids, and the heavy metals are solubilized in the catholyte [59]. However, the EDS process is time consuming [57,58,60,61], and the operating costs are relatively high [52,57,62].
Table 2. Summary of the extractants used to extract P, Zn, Pb, Cu, Cr, and/or Cd from selected ashes (marked with the x) with corresponding extraction rates (in %). The analyzed literature does not contain such extraction rates for WBA.
| Extractant | Conc. (mol/L) |
P | Zn | Pb | Cu | Cr | Cd | SSA | MSWI | WBA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | 0.05 | >95 | x | [22] | |||||||
| 0.1 | 88.3 | x | [24] | ||||||||
| 0.19 | 100 | x | [23] | ||||||||
| 0.19 | ~88 | x | [23] | ||||||||
| 0.2 | 92 | x | [2] | ||||||||
| 0.2 | 94 | x | [38] | ||||||||
| 0.25 | 93 | ~36 | ~1 | ~38 | ~5 | ~28 | x | [4] | |||
| 0.4 | 96.4 | x | [37] | ||||||||
| 0.5 | >70 | ~42 | 38.4 | ~40 | 57.7 | 50 | x | [39] | |||
| 0.5 | 74 | ~42 | ~40 | x | [25] | ||||||
| HNO3 | 0.3 | 89 | ~32 | ~24 | ~36 | ~5 | ~27 | x | [4] | ||
| 0.4 | 100 | x | [37] | ||||||||
| 0.5 | >70 | ~36 | 40 | ~38 | ~52 | ~6 | x | [39] | |||
| 0.5 | ~71 | ~36 | x | [25] | |||||||
| 1.5 | ~80 | 16 | 56 | x | [23] | ||||||
| 1.5 | 100 | 71 | 47 | x | [23] | ||||||
| HCl | 0.3 | 98.8 | ~32 | ~30 | 40 | ~5 | ~28 | x | [4] | ||
| 0.5 | >95 | x | [41] | ||||||||
| 1.0 | 75 | 1 | 71 | x | [40] | ||||||
| 1.0 | ~58 | ~1 | 40 | x | [15] | ||||||
| HCl + H2O2 | 1.0 + 9.8 | 68 | 62 | 97 | x | [40] | |||||
| Citric acid (C6H8O7) |
0.2 | ~80 | x | [38] | |||||||
| 0.4 | 59.3 | x | [37] | ||||||||
| 0.5 | >70 | ~23 | 13.3 | ~16 | ~25 | ~7 | x | [39] | |||
| 0.5 | 72 | ~23 | ~16 | x | [25] | ||||||
| Oxalic acid (C2H2O4) |
0.05 | 100 | x | [22] | |||||||
| 0.2 | >95 | x | [38] | ||||||||
| 0.55 | 95.4 | 37 | ~1 | 37 | ~8 | ~13 | x | [4] | |||
| 0.4 | 100 | x | [37] | ||||||||
| 0.5 | >70 | 56.9 | 4 | 65.8 | ~53 | ~13 | x | [39] | |||
| 0.5 | 74 | 56.9 | 65.8 | x | [25] | ||||||
| Lactic acid (C3H6O3) |
0.4 | 28.4 | x | [37] | |||||||
| EDTA (C10H16N2O8) |
0.02 | ~20 | x | [38] | |||||||
| 0.05 | <30 | ~14 | 37 | ~5 | ~42 | ~6 | x | [39] | |||
| 0.05 | ~24 | ~14 | ~5 | x | [25] | ||||||
| 0.05 | ~40 | x | [38] | ||||||||
| EDTMP (C6H20N2O12P4) |
0.05 | <30 | ~10 | ~22 | ~9 | ~26 | ~6 | x | [39] | ||
| 0.05 | ~13 | 10 | ~9 | x | [25] | ||||||
| 0.05 | ~25 | x | [38] | ||||||||
| NaOH | 0.5 | 40 | ~3 | ~3 | ~2 | ~2 | ~4 | x | [4] |
2.2. Methods Applied on an Industrial Scale
Prototype plants are already implemented or under construction (i.e., at TRL7) for the wet extraction of P from SSA (EasyMining, Uppsala, Sweden), salts and Zn from MSWI ashes (Stena Recycling, Gothenburg, Denmark), Zn from MSWI ashes (RENOVA, Göteborg, Sweden) and the full-scale commercially available process FLUWA/FLUREC operating in Switzerland for the recovery of metals. Additional plants recovering P from SSA using H3PO4 are operating in Germany, Switzerland, and Japan. These wet extraction processes at the high TRL level are based on using waste acid from nearby industries or from the wet scrubber of the incineration plant itself. A wet extraction method using HCl and lime for recovering commercial P, Fe, and Al products called Ash2Phos was developed by EasyMining, Sweden. The process has recovery rates of 90–95% for P, 60–80% for Al, and 10–20% for Fe from SSA [63]. Simultaneously, the heavy metal content in connection to P is reduced by at least 96%, making it a very pure and clean fertilizer product [63]. SSA is dissolved in HCl at 40 °C, and the P, Fe, and Al are separated as pure Ca3(PO4)2, FeCl3, and NaAlO2 [63]. The separation process is based on chemical precipitation steps in a unique combination, and the solution is later neutralized to remove heavy metals. CaO is used during the precipitation steps and for neutralization. The produced phosphorus–calcium-rich product (Ca5(PO4)3OH) contains a minimum of 16.5% P and 35% Ca and can be used as raw material for feeds or fertilizer applications [63]. The Fe and Al products can be reused in wastewater treatment plants [63]. After the treatment, non-dissolved SSA is filtered, washed, neutralized, and called “silicate sand” (48.3% SiO2, 22.9% Fe2O3, 7.2% Al2O3), which is potentially useable as a partial cement replacement in mortars after milling [64]. A full-scale plant able to annually treat 30,000 tons of SSA is under construction in Sweden, and plants in Germany are also under development [63].
The recovery of metals from MSWI-FA is achieved by the FLUWA/FLUREC processes developed in Switzerland by AIK Technik AG, as well as by the HaloSep process in Denmark developed by Stena Recycling. In 2018, >60% of the MSWI-FA in Switzerland was treated with the FLUWA process [39], which is based on wet extraction by adding acidic (HCl) and neutral (NaCl) waste scrub water to MSWI-FA where 60–80% Zn, 80–95% Cd, 50–85% Pb, 50–85% Cu can be extracted [40,65]. The metal-enriched filtrate obtained after FLUWA needs to be further processed to recover the metals, either by leading the filtrate to a wastewater treatment plant to precipitate a metal hydroxide sludge that can be recovered in smelting plants or by the FLUREC process, which allows a high-purity Zn recovery. Cd, Pb, and Cu are separated by reductive separation (cementation) using Zn powder as a reducing agent [65]. This cement, with a high Pb content of 50–70%, can be sent directly to a lead smelter where metals are recovered in the Pb production process [65]. Zn is removed from the remaining liquid by solvent extraction, followed by electrowinning to recover high-grade Zn (>99.99% Zn), which can be sold [66]. The remaining FA particles (filter cake) are currently landfilled.
The HaloSep process is another wet extraction method using a HCl scrubber liquid and MSWI-FA, which produces brine and a neutralized and washed FA. The resulting residues from the process are a stabilized FA, a metal fraction, and a brine solution. The metals are precipitated from the brine into a filter cake containing up to 38–40% Zn, which can be recovered at smelters. The remaining brine contains salt products (CaCl2, NaCl, KCl) useful for industrial applications. The treated FA complies with the European leaching limits for acceptance in landfills [65] but can also be used in construction [67]. A full-scale HaloSep plant is operating at the incineration plant Vestforbrænding in Copenhagen, Denmark, and plants in other countries are under exploration [67].
The RENOVA process also uses a HCl scrubber liquid for Zn leaching but differs from HaloSep by using an acidic pH in the process and re-incineration of the leached ash to destroy dioxins. Up to 70% Zn was leached in pilot scale studies, and NaOH-precipitated filter cake contained 80% Zn(OH)2 [68]. Re-incineration studies showed that more than 90% of the leached ash was converted into bottom ash [68]. There are plans to build a demonstration plant in Sweden [69].
Additional plants recovering P from SSA using H3PO4 are, e.g., a sewage sludge incineration plant in Werdohl, Germany, which uses the Remondis TetraPhas process [52]. It consists of leaching P from SSA by H3PO4 and purifying the P-concentrated acid leachate, allowing an 80% P extraction. The product, called RePacid, mainly contains H3PO4 and can be directly used by the industry [52]. Another solvent-extraction process called Phos4life was designed in the canton of Zürich, Switzerland, where the main product is technical H3PO4 (74%). Here, P is extracted from SSA by H2SO4, and more than 95% of it can be recovered from SSA in the form of H3PO4 [52]. Another well-known P production company is Nippon Phosphoric Acid Co., Ltd. (NPA) in Achi, Japan, where the H3PO4 is also obtained through a wet extraction process followed by filtration and purification. Gypsum (CaSO4·2H2O), with possible applications in cement, plasterboard, or soil improvement, is a by-product of this process [52].
2.3. Economic Assessment of Alternative Extraction Methods
In addition to achieving an optimal environmental profile for the extracted resources, the economic costs of recovering secondary raw materials are also of great importance, as both environmental and economic costs influence their acceptance in society [35]. Although high percentages of P extraction can be achieved by acid wet extraction of P, the acid demand is very high [70]. An example of acid demand is given by Donatello et al., where an optimized procedure showed that 368 kg of 98% H2SO4 was required for 80–100% P extraction from 1 ton of SSA [71]. Handling and transporting such large quantities of acid is not easy in all urban environments [70]. Moreover, the simultaneous extraction of heavy metals and P [71] requires a second separation step to support the European Green Deal, which promotes the recycling of limited resources while emphasizing the goal of zero pollution [70]. For this purpose, the development of alternative technologies, such as thermochemical methods, is supported. Products recovered by thermochemical methods are still ash, and the separated heavy metals represent only a small part of the ash, which is suitable for transport [72]. However, the energy consumption and high capital costs of the alternative methods still need to be optimized compared to the traditional wet chemical method [3,34,36]. In 2016, Egle et al. reported that the cost of recovering P from SSA using the wet chemical method is about 5–6 EUR/kg of P, whereas using the thermochemical method, the cost is about 2–3 EUR/kg of P [73]. However, the thermochemical method is closely related to the energy price, and with the current increasing energy prices, the cost of the thermochemical method has certainly increased [72]. Therefore, alternative extraction methods, such as microwave-assisted acid extraction, can reduce the total cost by up to 76% for MSWI-FA and up to 52% for MSWI-FA compared to traditional metal extraction by heating [35]. This cost analysis considered the cost of acid/chemicals, energy consumption, miscellaneous costs, and other laboratory costs for processing a given amount of ash [35]. In addition, alternative technologies, such as electrochemical methods, are still in the development and optimization phase, so there are not many analyses of their economic performance.
3. Potential Uses of Solid Residues of Ashes
Waste incineration is steadily increasing in Europe, but there are environmental concerns about the solid residues that require pre-treatment and are usually landfilled [74]. There are possibilities to use as-received or pre-treated ashes in agriculture, soil stabilization, and the building sector as the following:
- -
-
Supplementary cementitious materials (SCMs);
- -
-
Precursors for alkali-activated materials (AAMs);
- -
-
Artificial fillers or fine aggregates;
- -
-
Additives in clay-based materials;
- -
-
Precursors for carbonated products.
The use of waste ash in construction materials has attracted many researchers in recent years [74]. However, a good understanding of their chemical, physical, and microstructural characteristics is necessary for their full-scale use [75]. The question of how to keep the ash characteristics constant when heterogeneous materials such as sewage sludge, MSW, or varying types of biomasses are incinerated is especially important for their large-scale utilization.
AAMs/geopolymers are synthetic materials obtained by alkaline activation of Si- and Al-rich materials and specific industrial wastes [76]. AAMs are alternative cementitious or ceramic-like binders used as alternative construction materials and for the solidification/stabilization of various waste streams [77]. As cement production is among those human activities most generating CO2 emissions, sustainable development of the building industry requires three approaches: using renewable energy, using recycled products, and replacing cement [78]. Every 600 kg of cement causes about 400 kg of CO2 to be released into the atmosphere [79,80,81,82,83]. Therefore, the potential applications of different ashes as SCMs have been studied for decades [84] but recently with a focus on bio-based ashes [80].
Another very promising research topic in the cement and concrete industry is carbonation utilizing CO2 sequestration: a low-tech approach to the carbon capture and storage process that mainly involves the reaction of CO2 with Ca-containing materials to form Ca carbonates [85,86,87]. Potential sources from waste streams are ashes containing a certain amount of Ca and Mg compounds, especially WBA [88,89,90].
3.1. Potential Use of SSA
Sewage sludge is the most common and continuously generated by-product of wastewater treatment, containing the 2nd highest amount of P after bone meal [3,5,47]. It has a great potential for P recovery after an appropriate thermal treatment [2,4,22,23,24,25,37,52,53]. Sewage sludge has been directly used as agricultural fertilizer for decades, but its limitations are increasing all over the world due to the high contents of heavy metals, organic pollutants, and micro/nanoplastics. Hence, its incineration is considered to be the best way for disposal [53]. The incineration of sewage sludge at about 850 °C is widely used in the EU and is currently the most efficient method, reducing the volume by 90% (the mass by 70%) and removing organic pollutants and pathogens [47,52,59]. The resulting SSA contains 4–12 wt% of P, usually in the form of AlPO4 and Ca3(PO4)2, which are poorly bioavailable [26]. SSA also contains Fe and potentially toxic trace elements such as Zn, Pb, Ni, Cr, and Cd and is mostly landfilled [5,23,48]. Pre-treatment is required to prevent the loss of this potential P source and aims to increase the bioavailability of P and remove heavy metals, which often exceed the legal limits for fertilizer production, see Table 3 [24,48,74,91]. With, for example, innovative EDS, 80–90% of the P can be recovered while also achieving a low content of heavy metals [58,59,60]. A high concentration of CaO and SiO2 in the SSA after P extraction is the main reason for using SSA as a building material component [5].
Table 3. Legal limits for trace elements in EU fertilizing products (in mg/kg), adapted from the EU regulation [86].
| Element | Organic Fertilizer | Organo-Mineral Fertilizer | Inorganic Fertilizer |
|---|---|---|---|
| As | 40 | 40 | 40 |
| Cd | 1.5 | 3 | 3 |
| Cr | 2 | 2 | 2 |
| Cu | 300 | 600 | 600 |
| Hg | 1 | 1 | 1 |
| Ni | 50 | 50 | 100 |
| Pb | 120 | 120 | 120 |
| Zn | 800 | 1500 | 1500 |
SSA is a material comparable to lightweight sand and is less dense than Portland cement [47]. It consists of porous particles with irregular shapes, which is not ideal for its classification as a potential cementitious material [47]. It should be noted that the extraction of P with H2SO4 produces CaSO4, which negatively affects the cement properties [4]. Using OA as an extractant produces Ca oxalate, which does not have this negative effect [4]. SSA typically contains an elevated amount of about 14% Al2O3 compared to the ca. 5% in Portland cement, indicating a natural suitability for use in aerated concrete [47]. The high Al2O3 content in SSA may also benefit the chloride attack resistance in concrete applications due to the chloride binding capacity of amorphous Al2O3 [47]. SSA can be used as a possible cement replacement material, but it requires a pre-treatment due to the undesirable effects of the contained heavy metals and P recovery. It has the potential to replace cement in mortars [23,92] or partially replace clay in bricks [59]. Due to the small grain size, SSA is also suitable as a filler or fine aggregate component in mortar and concrete, where the effects on strength performance have been shown to be manageable for SSA contents up to 15 wt% [47,49,93]. Research on the reuse of SSA as an aluminosilicate precursor material for alkali activation/geopolymerization has also recently begun [77,94,95,96].
3.2. Potential Use of MSWI Ashes
MSW contains wood but also paper, plastic, glass, and textile scrap material, which cannot be degraded naturally. In the last few decades, the total mass of MSW has increased drastically due to rapid urbanization and an increased world population. This has encouraged many countries to properly dispose of this waste [74,97]. The so-called “green economy” has begun, encouraging waste reduction, reuse of materials through recycling or recovery, and supporting sustainability [97,98]. Several different treatments of MSW have been developed, as shown in Figure 2. The gases produced by the natural decomposition of MSW in landfills represent 18% of the energy production from biogas in the EU [97,99]. Nowadays, one effective and popular method is the incineration of MSW due to the volume reduction in MSW by 90% recovery of heat/energy. Two main residues are produced by incineration: around 80 wt% MSWI-BA and around 20 wt% MSWI-FA [27,51,97,100,101,102].
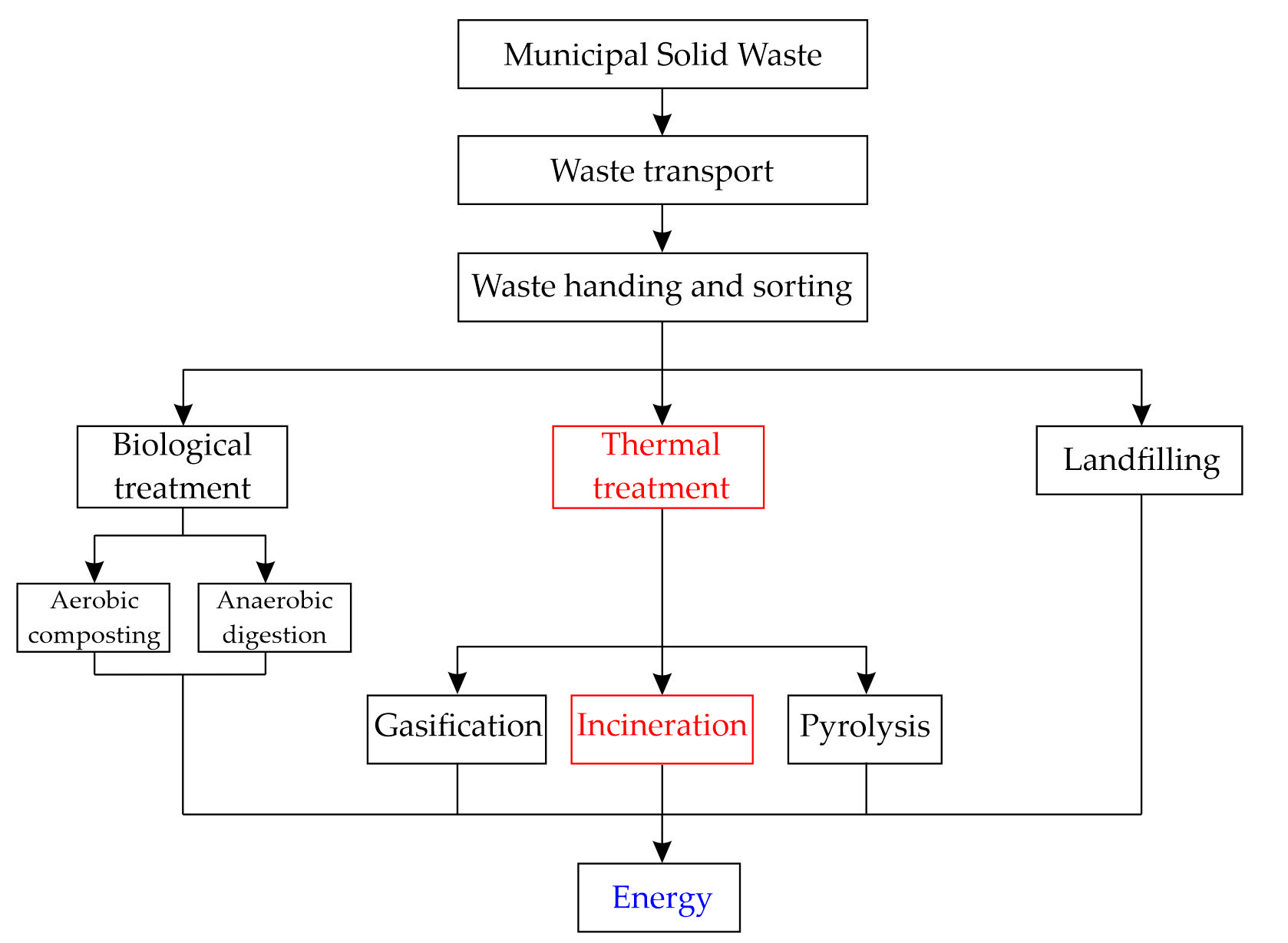
Figure 2. Different treatments for MSW management; redrawn based on [97].
MSWI-BA is classified as non-hazardous waste and mainly consists of amorphous SiO2, Al2O3, and CaO; its exact composition varies from incineration plant to incineration plant and even from batch to batch within a single incineration plant [97,100]. MSWI-BA is commonly utilized in road construction [103] and can be an alternative lightweight aggregate [97] or an alternative material for cement production [104,105]. One of the advantages of using MSWI ash as cement raw material is the reduction in CO2 emissions [104]. MSWI-FA is classified as a hazardous waste as it contains soluble salts, dioxins, and a significant amount of heavy metals such as Zn, Pb, and Cd [40]. Due to the presence of potentially leachable contaminants harmful to the environment and human health, landfill sites are becoming fewer, and the possibility of utilizing MSWI-FA has attracted many researchers [27,35,50,51,61,101,102,106]. Sekito et al. reported a 2-fold higher content of Zn and Pb in MSWI-FA compared to MSWI-BA, while the content of Cd was even 13-fold higher [107]. Therefore, MSWI-FA must be pre-treated before further use, and much research has focused on how to extract and recover various metals from it. In MSWI-FA, the pH has a significant effect on the removal efficiency of heavy metals [50]. Many metals have a high solubility at low pH levels, so using strong acids as the extractant is necessary. As MSWI-FA is alkaline, alternative methods are desired to avoid the consumption of large acid volumes. A new microwave-assisted acid extraction method has recently been developed [35]. Significant advantages of this method are lower costs, shorter processing times, and better efficiency of metal extraction compared to conventional heating [35,108,109,110].
Electrodialytic (ED) treatment is another innovative method also used for contaminated SSA [59]. It reduces the content of heavy metals and salts and increases the reactivity of Si and the Si/Al ratio [61,111]. Such a pre-treatment method can make MSWI-FA into a potential precursor in geopolymers based on AAMs that can naturally trap heavy metals inside its matrix. As MSWI-FA is alkaline (having a pH of around 11), ED treatment results in an acidic pH, similar to the common natural precursor in geopolymers, i.e., metakaolin. The combination of MSWI-FA pre-treatment and initiating up to 20 wt% of MSWI-FA in geopolymers achieves the lowest metal leaching and a high compressive strength, making it a potential construction material [61,111]. Thus, the use of geopolymerization for hazardous waste not only contributes to the best technological practices and legal provisions but is also ecologically efficient [112]. Studies have shown that the use of raw MSWI-FA is also possible but achieves lower compressive strengths than that of AAMs prepared with slag or pulverized FA [101]. The reason for the low compressive strength could be its variable mineral composition because MSWI-FA contains less SiO2 and Al2O3 than slag or pulverized FA and has a large specific surface area due to a high proportion of small particles [101].
MSWI-FA has also been studied as a potential replacement for cementitious materials, but adding it to cement-based products means that technical and environmental requirements such as sufficient strengths, disabilities, and leaching limits of heavy metals must be met [113]. The main problem with using MSWI-FA as a cement substitute is the presence of leachable toxic heavy metals and a high salt content. It is beneficial to use water washing and mechanochemical [113] or ED pre-treatments [114] to improve its performance before it is used in mortar, concrete, or bricks. The mechanochemical processes can stabilize the heavy metals and activate the MSWI-FA, allowing it to partially replace Portland cement in building materials [113], while an ED treatment can remove heavy metals and soluble salts from the MSWI-FA suspension which is thus decontaminated [96,115]. As an ultrafine material, MSWI-FA is also a potential substitute for clay in bricks, which should stabilize the heavy metals, reduce raw material imports, and, at the same time, conserve primary clay resources [115]. Studies have shown that fired bricks with an addition of 2.5–5 wt% treated MSWI-FA may be feasible [96,116,117].
3.3. Potential Use of WBA
WBA results from wood biomass combustion generating WB-BA, collected from the bottom of a combustion chamber, and WB-FA, which is subdivided into fine fly ash (particle size <1 µm, collected from electrostatic or bag house filters) and coarse fly ash (particle size >1 µm, collected from the cyclone or boilers) [83,118,119]. CaO and SiO2 are generally the major chemical components in WBA, while other compounds such as Al2O3, Fe2O3, K2O, Na2O, MgO, P2O5, or SO3 occur in lower amounts [15,75,120]. Minor contents of As, Cd, Cr, Cu, Pb, and Ni have also been detected [15,75]. Significant differences in the content of volatile heavy metals occur amongst the ash types and are the main concern when using WBA. A higher concentration of heavy metals was measured in WB-FA compared to WB-BA, as heavy metals are more concentrated in smaller particle size fractions (<75 µm) [12,14,118,119]. The particles of the fine fly ash fraction are also lighter and smaller, making them easy to inhale and a health risk, e.g., Cd accumulates in the kidneys and affects bone density [121].
The chemical composition of WB-FA also differs from coal FA, as WB-FA usually contains more alkali elements and less Al [118]. Nutrients, such as P and Mg, are primarily found in the WB-BA and coarse WB-FA. WB-FA shows significantly lower Cd concentrations compared to MSWI-FA [12,15,28,29]. The chemical and physical properties of WBA depend on the combustion technology, the heat treatment temperature, the tree species, and the geographic location; however, other factors, such as soil conditions, climate characteristics, and storage methods, also influence its properties [12,13,14,15,82,83,118,121,122].
Since wood biomass is considered to be a CO2-neutral renewable energy source, it is environmentally desirable to use WBA in the construction industry [119,123]. This would not only reduce rising disposal costs, 70% of WBA still ends up in landfills, but also preserve natural resources and reduce greenhouse gas emissions [119,124]. WBA has the potential to be used in various construction areas: as a partial replacement of aggregates or mineral admixtures in concrete [118,124], as a partial replacement of raw materials for clinker [118,119], as a filler/partial sand replacement material in cement-based materials [13,28], in brick production [13], road construction [13] and others. Proper storage and transport conditions are important for WBA use in cementitious composites, as carbonation and hydration can occur suddenly during these procedures in wet circumstances and thus strongly determine the quantity of CaO and other carbonate elements [13]. It is also very important to find the optimal cement/ash ratio so that the strength of the cement composites remains sufficient [13,125]. Replacing up to 45% of cement with WB-FA has been described as suitable for construction purposes; however, WB-FA has more potential as a filler material than as a cement replacement material in construction [13,82]. Most studies report that the optimal content of WB-FA and WB-BA to replace part of the cement in mortars is 10 wt% [13,79,80,126]. It has been reported that adding WBA generally lowered the mechanical properties of bricks [127,128], but many clay mixtures still fulfilled the required strength parameters. Another emerging use of WBA is as a feedstock material for CO2 utilization via mineral carbonation [129,130]. Even though the CO2 uptake capacity is limited, it can be improved as, e.g., mechanochemical activation has doubled its capacity [131]. Biomass ash is also a low-cost medium that does not need to be transported to a plant and can be used for a low-tech sequestration approach [86].
The suitability of coal combustion FA for the production of AAMs has been proven many times due to its suitable chemical composition and the large volume available [132,133]. It is influenced by the coal properties, the combustion technique, and the waste handling. WB-FA can similarly be used as a complete or partial replacement material when preparing geopolymer mortars, which could reduce the cost of geopolymer source materials and avoid the cost of WBA landfilling [76,132]. Replacing coal combustion FA by up to 20% WBA in the binder mass resulted in better strength and porosity properties than the control mixture after 3 and 7 days of aging. After 28 days, the geopolymer containing 10% WBA was the only one to show a higher compressive strength and a lower total porosity than the control mixture [132].
Using WBA as a forest fertilizer also has potential; however, future research should focus on the effect of trace element solubility on the natural leaching processes in forest soil [12,134]. Another important factor preventing the use of WBA on certain soil typologies is its alkaline pH (usually higher than 12) [135]. Accordingly, Pasquali et al. proposed a technology to stabilize heavy metals in WBA and lower their pH based on the use of other by-products (coal FA, rice husk ash, and MSWI-FA) [135]. MSWI-FA has a similar pH as WBA and is a source of leachable heavy metals, while its P concentration is low. Ca-rich coal FA was used in the stabilization procedure, while rice husk ash was chosen as a heavy metal stabilizer due to its amorphous silica content. Wolffers et al. recently reported the recovery of heavy metals from WB-FA based on acid leaching, a process also applied to MSWI-FA [15]. In Switzerland, the disposal of WB-FA in landfills will be prohibited in 2023 due to the elevated concentrations of very toxic Cr(VI) and other heavy metals [15]. The FLUWA process represents a promising method for managing WB-FA [15].
This entry is adapted from the peer-reviewed paper 10.3390/ma16216948
This entry is offline, you can click here to edit this entry!
