Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chitosan has received much attention for its role in designing and developing novel derivatives as well as its applications across a broad spectrum of biological and physiological activities, owing to its desirable characteristics such as being biodegradable, being a biopolymer, and its overall eco-friendliness.
- chitosan (CS) derivatives
- antimicrobial activity
- drug delivery
1. Introduction
Over the last few decades, natural polymers, including biopolymers, polyesters, and proteins, have displayed diverse applications in the fields of agriculture, pharmaceuticals, food safety, and the cosmetic industry due to their biodegradability, eco-friendliness, compatibility, and cost-effectiveness [1][2][3]. As a result, natural polymers have gained significantly increased interest in creating novel drugs, owing to their distinct and intricate chemical composition, which is responsible for their wide and diverse range of biological effects [4][5]. Among these, chitosan (CS) stands out as a widely utilized polysaccharide that has been extensively studied across various scientific disciplines [6][7][8]. CS is composed of linear polysaccharides with varying amounts of (β1 → 4) linked residues of N-acetyl-2 amino-2-deoxy-D-glucose (glucosamine, GlcN) and 2-amino-2-deoxy-D-glucose (N-acetyl-glucosamine, GlcNAc) residues distributed inside the polymer. It is derived from chitin, polyβ-[1,4]-N-acetyl-d-glucosamine) (or poly(N-acetyl-d-glucosamine) via deacetylation in the presence of sodium hydroxide or enzymes (Scheme 1) [9][10][11]. Chitin is found in crustaceans, including crabs, shrimp, shellfish, and lobsters, and its extraction primarily involves demineralization, deproteinization, and discoloration [12][13]. Its hydrophilic and reactive properties are limited by the presence of acetyl groups (CH3CO−) attached to the amino groups of GlcNAc. These acetyl groups function not only as barriers for water molecules, but also as protective shields, which reduce the availability of the amino groups for reactions [14]. For the biological and physical activity of CS, the degree of deacetylation (DD) is the pivotal factor, which represents the proportion of β-1,4-D-glucosamine repeating units in the polysaccharides during the deacetylation process originating from chitin [9][10][11][14]. A higher DD denotes an increased presence of free amino groups, which can form ammonium (NH4+) groups within a pH range of 5–6, resulting in increased water solubility and the enhancement of the biological impact of CS [15]. Furthermore, a lower molecular weight (MW) is another factor that decreases with a higher DD, which impacts the bioactivity of CS [15]. CS with a lower MW has demonstrated antibacterial, antioxidant, and anti-tumor properties due to its increased water solubility, ease of permeability to cells, and binding efficacy to DNA or RNA [16][17].
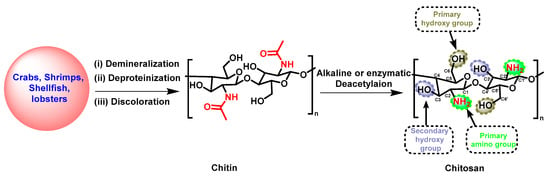
Scheme 1. Synthetic strategy for the preparation of CS.
CS is a cationic biopolymer agent that can interact with negatively charged substances such as lipids, fats, ions, cholesterol, proteins, and other organic and inorganic molecules [18]. CS was first identified in 1811 in mushrooms. Extensive research has revealed that CS significantly exhibits antimicrobial, antiviral, anticancer, non-toxic, water treatment, biocompatible, and biodegradable properties [19][20]. However, its solubility in acidic conditions (pH less than 6) limits certain bioactivities and applications. To address these issues and to enhance the bioactive and physiochemical effectiveness under neutral and physiological conditions, the modification of CS is a promising alternative since it has three active functional groups: C2-NH2, C3-OH, and C6-OH (Scheme 1) [21]. The nucleophilic substitution reactions involving the amino and hydroxyl groups, followed by multi-step reactions, have been studied, including N-alkylation/amination, acetylation, cross-linking, oxidation, grafting, and sulfation [22][23]. Synthetic reactions are accomplished through chemical or enzymatic catalysis, polymerization, or hydrolysis conditions to achieve the desired product [24]. The CS derivatives, which vary in terms of their degree of substitution (DS), a crucial factor in the structure-activity relationship (SAR), demonstrate unique physiochemical characteristics. Novel scaffolds with these characteristics have enhanced biocompatibility, bioactivity, biodegradability, and non-toxicity while retaining the inherent pharmacological effects of their antibacterial, anticancer, and antiviral properties [25]. Moreover, CS derivatives have also been widely utilized in the field of nanomaterials due to their immense potential as carriers for targeted drugs, adjuvants, or vaccines, facilitating the treatment of various diseases including cancer, diabetes, Alzheimer’s, cardiovascular disorders, and inflammatory conditions [26][27]. These nanomaterials have shown superior outcomes compared to conventional drug formulations.
2. Applications of Chitosan Derivatives
Antimicrobial Actions of Chitosan
CS has garnered extensive attention across a wide spectrum of applications, ranging from its role as an antimicrobial agent to its utility as a drug carrier. Numerous research investigations have sought to unveil CS’s antimicrobial attributes against a diverse array of microorganisms, including bacteria, fungi, yeast, and algae, primarily due to its biocompatible, biodegradable, and non-toxic properties [20][28]. The antimicrobial effects of CS are rooted in its physicochemical characteristics, which encompass the degree of deacetylation, its structural advantage in possessing reactive hydroxyl groups at the C-3 and C-6 positions, environmental conditions, and the specific microorganism in question [28]. The native CS, chitin, exhibits limited antimicrobial efficacy due to the presence of acetylated repeating units, which restrain the exposure of free amino groups. Furthermore, it is highly hydrophobic in nature because of the strong intra- and intermolecular hydrogen bonding [29]. On the other hand, CS-bearing repeating units of β-(1 → 4)-2-amino-2-deoxy-β-D-glucose offer primary amine functionality, typically achieved through the deacetylation process. A higher DD in the CS during the deacetylation process signifies an increased presence of free amino groups. These amino groups can interact with microbial cell surfaces through electrostatic attraction. Additionally, CS’s solubility in acidic water allows it to form cationic polyelectrolytes, facilitating its easy interaction with microbial cells and leading to cell lysis and cell death [30]. High-molecular-weight and low-molecular-weight CS exhibit distinct actions against microbes. High-molecular-weight CS’s antimicrobial activity primarily resides in the extracellular compartment, as it cannot penetrate the cell wall. Here, it operates by altering cell permeability, resulting in a reduced nutrient uptake from the extracellular milieu and a diminished availability of essential metals due to chelation. In contrast, low-molecular-weight CS displays the capability to exert both extracellular and intracellular effects. This translates to alterations in mitochondrial function and consequential impacts on RNA and protein synthesis [31][32].
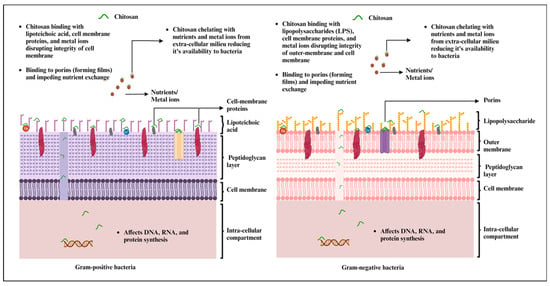
CS has undergone extensive testing against both Gram-positive and Gram-negative bacteria for antibacterial activity (Figure 1). However, the precise mechanism of action remains incompletely understood. Most studies underscore CS’s polycationic nature as a key contributor to its antibacterial efficacy [33]. Furthermore, its interactions with the bacterial cell wall are also dependent on the pH of the environment. When the environmental pH falls below CS’s pKa, electrostatic interactions occur between the polycationic CS and the anionic components of the bacterial cell wall. These components include lipopolysaccharides in the Gram-negative cell wall and certain proteins on the cell surface of bacteria. These interactions lead to the exertion of antibacterial effects [22][33]. Interestingly, the extent of electrostatic interactions is largely influenced by the number of amino groups linked to C-2 on the CS backbone. A higher number of amino groups results in more positively charged sites, enhancing the interaction with the carboxyl-negative charges on the bacterial cell wall [34].

Figure 1. Mechanism of antimicrobial effects of chitosan and chitosan-based materials against Gram-positive and Gram-negative bacteria (created with BioRender.com, accessed on 27 September 2023).
Chitosan Derivatives as Drug or Vaccine Delivery System
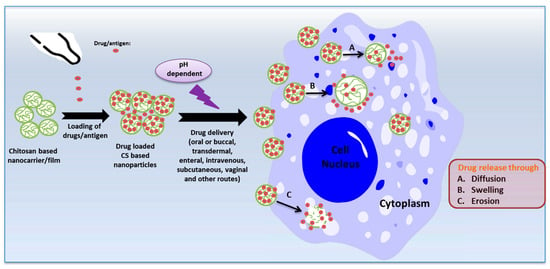
Controlled drug or vaccine release is highly emphasized as a strategy for controlling disease pathologies, achieved through the targeted accumulation of therapeutic compounds at the site of the ailment or the utilization of inventive therapeutic agents to control infectious pathogens [35][36]. Drug encapsulation and dosage reduction emerge as the most convenient methods for achieving controlled drug release [37]. CS and its derivative-based nanoparticles and films have received considerable attention as highly suitable candidates for drug or antigen carriers due to their mucoadhesive properties, pH sensitivity, and biocompatibility. The release of drugs/antigens from CS nanoparticles in biological fluids occurs through diffusion, erosion, or swelling mechanisms [38] (Figure 2). Additionally, their distinct antimicrobial activities collaboratively synergize with drugs to enhance their antimicrobial effectiveness [39].

Figure 2. The mechanisms of release of drugs/antigens from chitosan nanoparticles (created with Microsoft Power Point 2010 and Chemdraw 19.1).
The recent applications of CS-based nanomaterials as drug carriers against infectious diseases [40][41][42][43][44][45][46][47][48][49][50][51][52][53]. For example, the nasal delivery of hesperidin-loaded CS nanoparticles exhibited enhanced cellular uptake within inflammatory microenvironments and effectively suppressed cytokine storm syndrome and acute lung injury [40]. Meanwhile, CS biguanidine nanoparticles displayed remarkable inhibition against various Mycobacterium tuberculosis strains, including sensitive, multidrug-resistant (MDR), and extensively drug-resistant (XDR) types, surpassing the performance of the standard anti-tuberculosis drug, isoniazid [41]. Sawant et al. demonstrated that tuberculosis prevention could be achieved through the pulmonary delivery of rifampicin with improved solubility when encapsulated within octanoyl CS nanoparticles [42]. Additionally, CS-based materials proved effective in delivering various anti-infectious drugs and antibiotics, including carvacrol [43], doripenem [44], chlorhexidine [45], ciprofloxacin [46], dolutegravir [47], endolysin Cpl-1 [48], and amphotericin B [49]. These materials successfully inhibited the growth of pathogens such as Salmonella enterica, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, human immunodeficiency virus HIV, and fungal species. In the expanded application, the eradication of waterborne pathogens such as rotavirus and Shigella spp. was also investigated through CS-wrapped carbon nanotubes [50].
In highlighting the effort in the encapsulation process, the inactivated virus, RNA, proteins, and probiotics have also been capsulated in the CS derivatives, which serve as a nano-vaccine delivery system to increase the immune response against the pathogens [54][55][56][57][58][59][60]. As an instance, Tafaghodi et al. encapsulated the inactivated PR8 influenza virus within CS alginate and trimethyl CS nanoparticles which exhibited immunoadjuvant capabilities after nasal immunization, thereby stimulating a Th1-type immune response [54]. Inactivated dengue virus (DENV)-loaded CS nanoparticles displayed their high immunogenicity, inducing elevated levels of antibodies and T cells against DENV-2 in comparison with soluble DENV-2 immunogens [55]. CS-based nanoparticles, containing Salmonella Enteritidis outer membrane proteins and flagellin proteins, emerged as a mucosal vaccine, inducing specific immune responses and reducing cecal colonization in avian hosts [56].
The drug-release characteristics of CS nanoparticles exhibit intricate responsiveness to pH alterations, rendering them a versatile tool for precision drug delivery. Research indicates that alterations in pH levels induce significant shifts in CS nanoparticle size and drug molecule release. In a study incorporating 5-fluorouracil (5-FU) within CS nanoparticles, it was observed that as the pH transitions from 3 to 5, there is a substantial increase in particle size, resulting in a continuous and rapid release of 5-FU. Conversely, when the pH exceeds 5, a notable reduction in particle size is evident, particularly at pH 7.4. This pH-responsive behavior can be attributed to the protonation of primary amino groups within the CS chain, leading to heightened electric density and increased repulsion forces between interconnected CS chains. Consequently, the surface density of protonated amino groups and the degree of protonization are adaptably responsive to shifts in solution pH [61]. CS nanoparticles undergo a reversible process of swelling and contraction, contingent upon the environmental pH, resulting in particle sizes fluctuating from approximately 450 nm to 150 nm [62]. The pH-sensitive surface charge reversal also facilitates enhanced cellular uptake and endolysosomal escape, extending blood circulation time, mitigating side effects, and optimizing drug delivery efficiency [63]. Such adaptability holds significant promise for targeted drug delivery, aligning with the varying pH levels found within the human body, which include gastric juice with a low pH of 1–1.5, the physiological environment exhibiting a pH of 7.4, the tumor extracellular microenvironment typically with a pH 6.5, and the pH within endosomes and lysosomes ranging from 5.0–6.2 to 4.0–5.0, respectively [63][64].
Chitosan Derivatives in Plant Agriculture
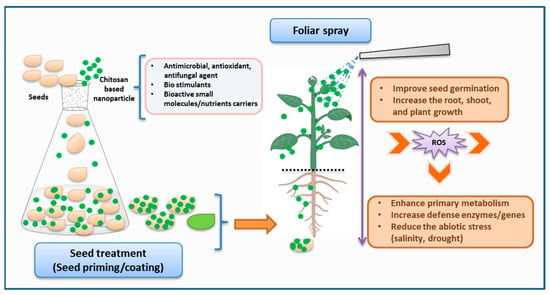
CS and its derivatives have gained significant interest in the agriculture field, especially for their role in enhancing plant growth, stimulating root and shoot development, increasing crop yield, and supporting environmentally friendly farming practices (Figure 3) [65]. The cationic property of CS derivatives enables effective interaction with the negatively charged components of the bacterial cell membrane, resulting in bactericidal or bacteriostatic effects [66]. On the other hand, the chelating characteristic of CS also establishes it as a tremendous antifungal agent [67]. Similarly, it has also demonstrated its role as an elicitor by activating the defensive genes in plant disease control [68].

Figure 3. Schematic representation of the application of chitosan-based nanoparticles in plant agriculture (created with Microsoft PowerPoint 2010, Chemdraw 19.1, and BioRender.com, accessed on 27 September 2023).
Considering the encouraging advantages, CS derivatives have been utilized in a wide range of crops, including vegetables, flowers, fruits, cereals, and medicinal plants, in various forms such as seed treatment, foliar spraying, coating fruits or vegetables, and delivering nutrients through nanomaterials [69]. For example, Udayashankar group investigated the CS exogenous seed priming of cucumbers seeds, resulting in the inducement of phytohormone production and an increment of resistance against cucumber powdery mildew disease [70]. Recent studies demonstrated the use of CS through seed priming, seed soaking, or seedling application in mung bean [71], Platycodon grandiflorus [72], and Carum copticum L. [73] for reducing abiotic stress and increasing photosynthesis and antioxidant activity, resulting in efficient plant growth. Similarly, plant diseases such as root rot caused by Fusarium solani in fenugreek were also managed with seed treatment [74]. CS derivatives such as CS–ferulic acid, CS–caffeic acid conjugate, and carboxymethyl CS were explored for seed treatment or seed coating in cucumbers [75] or Prunus davidiana [76]. As a result, they either increased the antioxidant activity or enhanced the biomass, photosynthetic capacity, and absorption of phosphorus and potassium to improve plant growth. In further applications, CS nanoparticles have been significantly introduced in seed treatment [77].
3. Conclusions
CS is considered a promising polysaccharide renowned for its extensive array of potential applications in the fields of biology, medicine, water treatment, the food industry, cosmetics, and biodegradable packaging. Certain constraints persist, such as a lower degree of deacetylation and elevated molecular weight, which directly result in poor water solubility in neutral aqueous solutions. The modification of CS through different strategic pathways is one perspective that not only improves the water solubility but also enhances the bioactivity of the final product. However, the CS-bearing long chain or hydrophobic scaffolds may diminish the water solubility; nevertheless, they can still be effectively incorporated into films, fibers, or polymeric materials to enhance the hydrophobicity and stability of the materials [78].
Exploring CS’s use as an antimicrobial agent unmasks a dynamic array of research possibilities, especially in the medical and agricultural fields. CS demonstrates significant bioactivity in combating both animal and plant pathogens. The research on nanoparticle engineering involving CS and its derivatives as drug carriers has significantly demonstrated the effective delivery of drugs or vaccines to target diseases in the pharmaceutical field. Antimicrobial dressings based on CS and its derivatives have received FDA approval [270,271]. However, new applications as drug carriers are still awaiting approval due to insufficient research on their mutagenicity and genotoxicity. Simple and mild preparation of CS-based nanoparticles is always encouraged for ideal drug delivery systems due to their water solubility. In addition, researchers have observed that treating seeds or foliar sprays by applying CS derivatives or nanomaterials demonstrated effective results in enhancing plant growth, increasing crop yields, and advancing food security. This approach not only reduces the need for pesticides and fertilizers but also contributes to environmental pollution reduction and promotes the sustainability of entire agricultural systems.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28227659
References
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337.
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Natural Polymers in Bio-Degradable/Edible Film: A Review on Environmental Concerns, Cold Plasma Technology and Nanotechnology Application on Food Packaging—A Recent Trends. Food Chem. Adv. 2022, 1, 100135.
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209.
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent Advances in Natural Polymer-Based Drug Delivery Systems. React. Funct. Polym. 2020, 148, 104501.
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539.
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan Based Bioadhesives for Biomedical Applications: A Review. Carbohydr. Polym. 2022, 282, 119100.
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-Based Hemostatic Hydrogels: The Concept, Mechanism, Application, and Prospects. Molecules 2023, 28, 1473.
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430.
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of Chitin from Prawn Shells and Conversion to Low Molecular Mass Chitosan. Food Hydrocoll. 2013, 31, 166–171.
- Harmsen, R.A.G.; Tuveng, T.R.; Antonsen, S.G.; Eijsink, V.G.H.; Sørlie, M. Can We Make Chitosan by Enzymatic Deacetylation of Chitin? Molecules 2019, 24, 3862.
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243.
- No, H.K.; Meyers, S.P.; Lee, K.S. Isolation and Characterization of Chitin from Crawfish Shell Waste. J. Agric. Food Chem. 1989, 37, 575–579.
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25.
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678.
- Li, X.; Xia, W. Effects of concentration, degree of deacetylation and molecular weight on emulsifying properties of chitosan. Int. J. Biol. Macromol. 2011, 48, 768–772.
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94.
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132.
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226.
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172.
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868.
- Argüelles-Monal, W.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342.
- Cohen, E.; Poverenov, E. Hydrophilic Chitosan Derivatives: Synthesis and Applications. Chem. Eur. J. 2022, 28, e202202156.
- Boominathan, T.; Sivaramakrishna, A. Recent Advances in the Synthesis, Properties, and Applications of Modified Chitosan Derivatives: Challenges and Opportunities. Top. Curr. Chem. 2021, 379, 19.
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880377.
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-Based Nanoparticles as Drug Delivery Systems: A Review on Two Decades of Research. J. Drug Target. 2019, 27, 379–393.
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465.
- Zia, K.M.; Barikani, M.; Zuber, M.; Bhatti, I.A.; Sheikh, M.A. Molecular engineering of chitin based polyurethane elastomers. Carbohy. Polym. 2008, 74, 149–158.
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272.
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-Based Nanosystems and Their Exploited Antimicrobial Activity. Eur. J. Pharm. Sci. 2018, 117, 8–20.
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502620.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Jeon, Y. Antimicrobial Effect of Chitooligosaccharides Produced by Bioreactor. Carbohydr. Polym. 2001, 44, 71–76.
- Alenzi, A.M.; Albalawi, S.A.; Alghamdi, S.G.; Albalawi, R.F.; Albalawi, H.S.; Qushawy, M. Review on Different Vesicular Drug Delivery Systems (VDDSs) and TheirApplications. Recent Pat. Nanotechnol. 2023, 17, 18–32.
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent Advances in Long-Acting Drug Delivery Systems for Anticancer Drug. Adv. Drug Deliv. Rev. 2023, 194, 114724.
- Lukova, P.; Katsarov, P. Contemporary Aspects of Designing Marine Polysaccharide Microparticles as Drug Carriers for Biomedical Application. Pharmaceutics 2023, 15, 2126.
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84.
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313.
- Jin, H.; Zhao, Z.; Lan, Q.; Zhou, H.; Mai, Z.; Wang, Y.; Ding, X.; Zhang, W.; Pi, J.; Evans, C.E.; et al. Nasal Delivery of Hesperidin/Chitosan Nanoparticles Suppresses Cytokine Storm Syndrome in a Mouse Model of Acute Lung Injury. Front. Pharmacol. 2021, 11, 592238.
- Abdallah, H.M.; Abu Elella, M.H.; Abdel-Aziz, M.M. One-Pot Green Synthesis of Chitosan Biguanidine Nanoparticles for Targeting M. tuberculosis. Int. J. Biol. Macromol. 2023, 232, 123394.
- Petkar, K.C.; Chavhan, S.; Kunda, N.; Saleem, I.; Somavarapu, S.; Taylor, K.M.G.; Sawant, K.K. Development of Novel Octanoyl Chitosan Nanoparticles for Improved Rifampicin Pulmonary Delivery: Optimization by Factorial Design. AAPS PharmSciTech 2018, 19, 1758–1772.
- Niaz, T.; Sarkar, A.; Mackie, A.; Imran, M. Impact of Albumin Corona on Mucoadhesion and Antimicrobial Activity of Carvacrol Loaded Chitosan Nano-Delivery Systems under Simulated Gastro-Intestinal Conditions. Int. J. Biol. Macromol. 2021, 169, 171–182.
- Yildiz-Peköz, A.; Akbal, O.; Tekarslan, S.H.; Sagirli, A.O.; Mulazimoglu, L.; Morina, D.; Cevher, E. Preparation and Characterization of Doripenem-Loaded Microparticles for Pulmonary Delivery. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 347–357.
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269.
- Abilova, G.K.; Kaldybekov, D.B.; Irmukhametova, G.S.; Kazybayeva, D.S.; Iskakbayeva, Z.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Chitosan/Poly(2-Ethyl-2-Oxazoline) Films with Ciprofloxacin for Application in Vaginal Drug Delivery. Materials 2020, 13, 1709.
- Priya Dharshini, K.; Fang, H.; Ramya Devi, D.; Yang, J.-X.; Luo, R.-H.; Zheng, Y.-T.; Brzeziński, M.; Vedha Hari, B.N. pH-Sensitive Chitosan Nanoparticles Loaded with Dolutegravir as Milk and Food Admixture for Paediatric Anti-HIV Therapy. Carbohydr. Polym. 2021, 256, 117440.
- Gondil, V.S.; Dube, T.; Panda, J.J.; Yennamalli, R.M.; Harjai, K.; Chhibber, S. Comprehensive Evaluation of Chitosan Nanoparticle Based Phage Lysin Delivery System; A Novel Approach to Counter S. pneumoniae Infections. Int. J. Pharm. 2020, 573, 118850.
- Zareshahrabadi, Z.; Khorram, M.; Pakshir, K.; Tamaddon, A.-M.; Jafari, M.; Nouraei, H.; Ardekani, N.T.; Amirzadeh, N.; Irajie, C.; Barzegar, A.; et al. Magnetic Chitosan Nanoparticles Loaded with Amphotericin B: Synthesis, Properties and Potentiation of Antifungal Activity against Common Human Pathogenic Fungal Strains. Int. J. Biol. Macromol. 2022, 222, 1619–1631.
- Pramanik, A.; Jones, S.; Gao, Y.; Sweet, C.; Begum, S.; Shukla, M.K.; Buchanan, J.P.; Moser, R.D.; Ray, P.C. A Bio-Conjugated Chitosan Wrapped CNT Based 3D Nanoporous Architecture for Separation and Inactivation of Rotavirus and Shigella Waterborne Pathogens. J. Mater. Chem. B 2017, 5, 9522–9531.
- Liu, L.; Ma, Q.; Wang, S.; Gao, Y.; Zhu, C.; Zhao, W.; Sun, W.; Ma, H.; Sun, Y. Efficient Epidermal Delivery of Antibiotics by Self-Assembled Lecithin/Chitosan Nanoparticles for Enhanced Therapy on Epidermal Bacterial Infections. Int. J. Biol. Macromol. 2022, 218, 568–579.
- Silva, M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.; Gonçalves, L. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370.
- Rashki, S.; Safardoust-Hojaghan, H.; Mirzaei, H.; Abdulsahib, W.K.; Mahdi, M.A.; Salavati-Niasari, M.; Khaledi, A.; Khorshidi, A.; Mousavi, S.G.A. Delivery LL37 by Chitosan Nanoparticles for Enhanced Antibacterial and Antibiofilm Efficacy. Carbohydr. Polym. 2022, 291, 119634.
- Mosafer, J.; Sabbaghi, A.-H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, Characterization and In Vivo Evaluation of Alginate-Coated Chitosan and Trimethylchitosan Nanoparticles Loaded with PR8 Influenza Virus for Nasal Immunization. Asian J. Pharm. Sci. 2019, 14, 216–221.
- Jearanaiwitayakul, T.; Sunintaboon, P.; Chawengkittikul, R.; Limthongkul, J.; Midoeng, P.; Chaisuwirat, P.; Warit, S.; Ubol, S. Whole Inactivated Dengue Virus-Loaded Trimethyl Chitosan Nanoparticle-Based Vaccine: Immunogenic Properties in Ex Vivo and in Vivo Models. Hum. Vaccines Immunother. 2021, 17, 2793–2807.
- Acevedo-Villanueva, K.Y.; Lester, B.; Renu, S.; Han, Y.; Shanmugasundaram, R.; Gourapura, R.; Selvaraj, R. Efficacy of Chitosan-Based Nanoparticle Vaccine Administered to Broiler Birds Challenged with Salmonella. PLoS ONE 2020, 15, e0231998.
- Helmy, Y.A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter Jejuni Infected Chickens. Infect. Immun. 2022, 90, e00337-22.
- Bento, D.; Jesus, S.; Lebre, F.; Gonçalves, T.; Borges, O. Chitosan Plus Compound 48/80: Formulation and Preliminary Evaluation as a Hepatitis B Vaccine Adjuvant. Pharmaceutics 2019, 11, 72.
- Zhao, K.; Li, S.; Li, W.; Yu, L.; Duan, X.; Han, J.; Wang, X.; Jin, Z. Quaternized Chitosan Nanoparticles Loaded with the Combined Attenuated Live Vaccine against Newcastle Disease and Infectious Bronchitis Elicit Immune Response in Chicken after Intranasal Administration. Drug Deliv. 2017, 24, 1574–1586.
- Hajam, I.A.; Senevirathne, A.; Hewawaduge, C.; Kim, J.; Lee, J.H. Intranasally Administered Protein Coated Chitosan Nanoparticles Encapsulating Influenza H9N2 HA2 and M2e mRNA Molecules Elicit Protective Immunity against Avian Influenza Viruses in Chickens. Vet. Res. 2020, 51, 37.
- Aydin, R.S.T.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 42.
- Lim, E.K.; Huh, Y.M.; Yang, J.; Lee, K.; Suh, J.S.; Haam, S. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv. Mater. 2011, 23, 2436–2442.
- Hu, X.; Wu, T.; Bao, Y.; Zhang, Z. Nanotechnology based therapeutic modality to boost anti-tumor immunity and collapse tumor defense. J. Control. Release 2017, 256, 26–45.
- Liu, H.; Liu, W.; Tan, Z.; Zeng, Z.; Yang, H.; Luo, S.; Wang, L.; Xi, T.; Xing, Y. Promoting Immune Efficacy of the Oral Helicobacter pylori Vaccine by HP55/PBCA Nanoparticles against the Gastrointestinal Environment. Mol. Pharm. 2018, 15, 3177–3186.
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801.
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009.
- García-Carrasco, M.; Valdez-Baro, O.; Cabanillas-Bojórquez, L.A.; Bernal-Millán, M.J.; Rivera-Salas, M.M.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Potential Agricultural Uses of Micro/Nano Encapsulated Chitosan: A Review. Macromol 2023, 3, 614–635.
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and Chitosan Activate Plant Defensive Genes through the Octadecanoid Pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098.
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing Sustainable Agriculture Systems in Medicinal and Aromatic Plant Production by Using Chitosan and Chitin-Based Biostimulants. Plants 2023, 12, 2469.
- Jogaiah, S.; Satapute, P.; De Britto, S.; Konappa, N.; Udayashankar, A.C. Exogenous Priming of Chitosan Induces Upregulation of Phytohormones and Resistance against Cucumber Powdery Mildew Disease Is Correlated with Localized Biosynthesis of Defense Enzymes. Int. J. Biol. Macromol. 2020, 162, 1825–1838.
- Hidangmayum, A.; Dwivedi, P. Effect of Chitosan Seed Priming on Mungbean Seedlings Subjected to Different Levels of Water Potential. Acta Physiol. Plant. 2023, 45, 6.
- Liu, H.; Zheng, Z.; Han, X.; Zhang, C.; Li, H.; Wu, M. Chitosan Soaking Improves Seed Germination of Platycodon Grandiflorus and Enhances Its Growth, Photosynthesis, Resistance, Yield, and Quality. Horticulturae 2022, 8, 943.
- Razavizadeh, R.; Adabavazeh, F.; Komatsu, S. Chitosan Effects on the Elevation of Essential Oils and Antioxidant Activity of Carum copticum L. Seedlings and Callus Cultures under in Vitro Salt Stress. J. Plant Biochem. Biotechnol. 2020, 29, 473–483.
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of Chitosan Seed Treatment of Fenugreek for Management of Root Rot Disease Caused by Fusarium Solani under In Vitro and In Vivo Conditions. 3 Biotech 2021, 11, 290.
- Nedved, E.L.; Kalatskaja, J.N.; Ovchinnikov, I.A.; Rybinskaya, E.I.; Kraskouski, A.N.; Nikalaichuk, V.V.; Hileuskaya, K.S.; Kulikouskaya, V.I.; Agabekov, V.E.; Laman, N.A. Growth Parameters and Antioxidant Activity in Cucumber Seedlings with the Application of Chitosan and Hydroxycinnamic Acids Conjugates under Salt Stress. Appl. Biochem. Microbiol. 2022, 58, 69–76.
- Xu, D.; Li, H.; Lin, L.; Liao, M.; Deng, Q.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H. Effects of Carboxymethyl Chitosan on the Growth and Nutrient Uptake in Prunus Davidiana Seedlings. Physiol. Mol. Biol. Plants 2020, 26, 661–668.
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662.
- Zhang, Z.; Jin, F.; Wu, Z.; Jin, J.; Li, F.; Wang, Y.; Wang, Z.; Tang, S.; Wu, C.; Wang, Y. O-acylation of chitosan nanofibers by short-chain and long-chain fatty acids. Carbohydr. Polym. 2017, 177, 203–209.
This entry is offline, you can click here to edit this entry!
