Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Heat shock factor 1 (HSF1) is a transcription factor crucial for cellular stress responses. HSF1 activates heat shock proteins (HSPs) in response to proteotoxic stress, aiding in protein folding and maintaining proteostasis. HSF1 is often overexpressed in various cancer cells, fueling malignancy and indicating a poor prognosis. The mechanisms behind HSF1-induced tumorigenesis are complex and cancer type-dependent. Targeting HSF1 presents a novel cancer treatment strategy.
- HSF1
- heat shock response
- cellular stress
- cancer stem cells
1. Introduction
The transcription factor heat shock factor 1 (HSF1) is crucial in regulating the heat shock response (HSR). HSR is a primary protective mechanism responding to stressful conditions such as elevated temperatures, oxidative stress, heavy metals, and proteotoxic insults [1][2]. When cells are exposed to proteotoxic stress, HSF1 induces the expression of heat shock proteins (HSPs) to act as chaperones, correcting the protein-folding process and maintaining proteostasis [2][3]. Beyond HSR, numerous studies demonstrate that HSF1 orchestrates transcriptional programs distinct from HSR and impacts cell proliferation, survival, and metabolism related to cancer [4][5][6]. HSF1 is overexpressed in multiple cancer types, and its activation supports malignancy and leads to poor prognosis [7][8][9]. As a result, HSF1 is a potential biomarker for identifying the malignancy of cells [10]. The mechanisms of HSF1-induced tumorigenesis are complex and involve diverse pathways, depending on the cancer type. Given the essential role of HSF1 in cancer, researchers are discovering the functions of HSF1 in tumorigenesis and developing HSF1 inhibitors as part of innovative targeted therapy [11].
HSF1 plays an important role in the progression of various cancer types, including those of the breast, lung, ovary, endometrium, and prostate and many other cancers. HSF1 has been reported to control critical oncogenic pathways, influencing cell cycle progression, apoptosis, and angiogenesis. Moreover, its potential to impact immunological responses, modulate the tumor microenvironment, and contribute to the development of therapeutic resistance highlights its importance in cancer biology.
2. HSF1 Biology
2.1. HSF1 Structure and Function
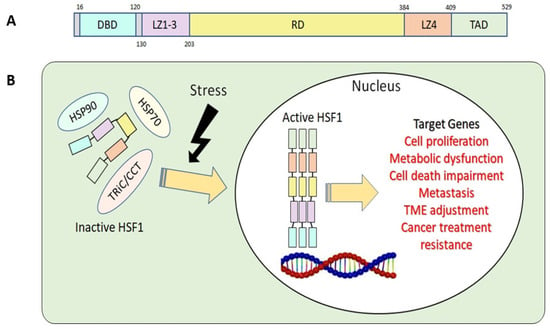
The structure of the HSF1 protein can be divided into five parts according to their functions: the DNA-binding domain (DBD), leucine zipper 1-3 (LZ1-3), the regulatory domain (RD), leucine zipper 4 (LZ4), and the transactivation domain (TAD) (Figure 1A) [12][13]. Under stress conditions, the N-terminal DBD binds to the target genes’ heat shock element (HSE) during HSR. This binding process requires HSF1 homotrimer formation and subsequent activation [14]. To avoid the continuous activation of HSF1, LZ1-3 and LZ4 form intramolecular interactions to keep HSF1 in its monomeric form and inactive [15]. RD, the domain between LZ1-3 and LZ4, provides an alternative way to regulate HSF1 positively or negatively via modification of specific amino acid residues, known as post-translational modification (PTM) [12][15][16]. Lastly, the C-terminal TAD is related to cell survival once cells undergo heat shock [17].

Figure 1. (A) Basic structure of human HSF1. DBD, DNA-binding domain; LZ1-3, leucine zipper 1-3; RD, regulatory domain; LZ4, leucine zipper 4; TAD, transactivation domain. (B) HSF1 activation related to tumorigenesis.
HSF1 binds to the HSE and functions as a critical regulator of HSR, triggering the transcription of genes encoding HSPs to prevent further damage by protein misfolding and aggregating [1][18]. However, recent studies discovered that, in addition to cytoprotective properties, continuous activation of HSF1 increases cell proliferation and survival and reprograms cell metabolism, similar to cancer cells [4][5][6]. Therefore, research focused on HSF1-induced tumorigenesis has increased dramatically.
2.2. HSF1 Regulatory Mechanisms in Normal and Cancer Cells
HSF1 undergoes regulation through various mechanisms, such as intrinsic regulation by LZ1-3 and LZ4 and interactions with chaperones/chaperonins. Additionally, it is subject to various post-transcriptional and post-translational regulatory processes. [12][15]. Furthermore, a recent study also indicated that HSF1 is regulated by non-coding RNA [19].
Upon activation of HSF1 due to stress, it assembles into homotrimers, moves from the cytosol to the nucleus, initiates HSP transcription, and activates HSR. In the nucleus, HSF1 binds to various target genes and activates their respective functions and roles (Figure 1B). As chaperones accumulate in response to HSF1 activation, they engage with HSF1, holding it in the cytosol as a monomer. This interaction attenuates HSRs by rendering HSF1 inactive [12]. This negative-feedback pathway prevents HSF1 overactivation [20].
The regulatory mechanism of HSF1 hinges on post-translational modifications, a process wherein changes occur to the protein after it has been synthesized, for example, phosphorylation [21][22][23][24][25], acetylation [26][27], and SUMOylation [28]. These modifications play a pivotal role in fine-tuning HSF1 activity, influencing its ability to form functional complexes, translocate to the nucleus, and trigger downstream cellular responses.
HSF1’s regulatory mechanisms can be changed in cancer cells, resulting in different patterns of activation and function. Even under non-stress settings, many cancer cells have a heightened and constitutive activation of HSF1. This persistent activation in cancer cells promotes survival and proliferation by increasing the expression of chaperone proteins and anti-apoptotic molecules, which aid in managing proteotoxic stress within rapidly growing malignant cells.
3. HSF1 Involvement in Various Cancer Types
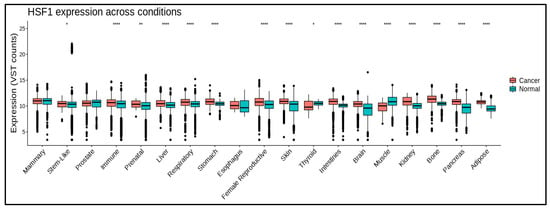
HSF1 promotes tumor progression and survival via a variety of methods. It controls gene expression during the cell cycle, apoptosis inhibition, tumor microenvironment modification, angiogenesis, and metastasis. HSF1 expression (Figure 2) is associated with poor prognosis and treatment resistance in various malignancies, including breast, prostate, lung, and ovarian cancers (Table 1). Some studies also suggest that HSF1 expression in tumor tissue also increases significantly according to clinical stage [29][30]. However, in some cancers, there are no significant correlation between HSF1 expression and clinical stage (Figure S1).

Figure 2. HSF1 mRNA levels in different cancers and normal tissues. HSF1 expression is significantly higher in some cancerous conditions. * means p ≤ 0.05; ** means p ≤ 0.01; **** means p ≤ 0.0001. Data are processed using https://gccri.bishop-lab.uthscsa.edu/correlation-analyzer/ (accessed on 10 October 2023).
HSF1 inhibition has emerged as a possible therapeutic strategy in cancer treatment. HSF1 inhibition has been demonstrated to sensitize cancer cells to chemotherapy, diminish tumor growth, and improve radiation therapy efficacy. Furthermore, HSF1 has emerged as a promising target for cancer therapy.
4. HSF1 in Therapeutic Resistance
While chemotherapy or radiotherapy remains the primary approach for treating various cancer types, the ongoing challenge lies in the development of drug resistance by cancer cells despite notable advancements in treatment. Multiple factors contribute to this resistance, with one of the factors being the overexpression or activation of HSF1.
Various cancer cells exhibit increased levels of HSF1, and this upregulation is linked to resistance against chemotherapy. Activated HSF1 boosts the production of HSPs, shielding cancer cells from the harmful impact of chemotherapy drugs. HSPs play a role in preventing protein misfolding and aggregation, aiding protein folding and breakdown, and inhibiting apoptosis—all contributing factors to the development of resistance to chemotherapy [28][31][32][33][34][35]. Cancer cells are stressed when subjected to chemotherapeutic drugs, which can activate HSF1 and the transcription of HSP70 and HSP90, further contributing to therapeutic resistance [36][37].
In addition to its role in HSP regulation, HSF1 also promotes tumor cell survival and proliferation by activating the expression of genes involved in cell cycle regulation [38][39][40], DNA repair [38][41], and angiogenesis [42][43]. Inhibition of HSF1 has been shown to sensitize cancer cells to chemotherapy and reduce tumor growth. Therefore, targeting HSF1 may provide a promising strategy for overcoming chemotherapy resistance in cancer treatment [44][45][46].
HSF1 is also a crucial factor in the transcriptional activation of multidrug resistance 1 (MDR1), which is involved in chemoresistance. The pivotal role of HSF1 in drug resistance can be demonstrated in the binding of HSF1 to the HSE of MDR1. Transfection of active HSF1 increases MDR1 mRNA and protein levels, stimulating drug efflux and the development of drug resistance [47][48]. On the contrary, HSF1 depletion downregulates the transcription of the MDR1 gene in the cells [49].
Another mechanism of multidrug resistance involves the ATP-binding cassette (ABC) transporters, which pump hydrophobic molecules out of the cell. The increasing efflux of drugs mediated by the ABC transporters is one of the most common mechanisms of drug resistance [50]. HSF1 promotes the activation of ABCB1, an ABC transporter. In melanoma cells with HSF1 overexpression, upregulation of ABCB1 gene transcription was prominent. The expression of the ABCB1 gene was found to be primarily dependent on HSF1 in all tested doxorubicin- and paclitaxel-resistant melanoma cell lines [51].
In addition to chemoresistance, HSF1 also plays a role in protecting cancer cells from the effects of radiotherapy by boosting the expression of HSPs [52][53][54]. Overexpression of HSF1 leads to radiotherapy resistance in cancer cells. HSF1 activation leads to the upregulation of genes involved in DNA repair, including Rad51, a protein involved in homologous recombination repair, a critical pathway for repairing DNA double-strand breaks induced by ionizing radiation [41][55][56]. Furthermore, HSF1 has been shown to regulate the expression of several anti-apoptotic genes, including Bcl-2 [57][58], which can protect cancer cells from radiation-induced apoptosis. HSF1 has also been shown to activate the NF-κB pathway [59][60][61], which regulates inflammation and immune responses. These three mechanisms may enhance radioresistance by inducing the expression of several pro-survival genes, including HSPs.
High expressions of HSP27, HSP70, and HSP90 exert a radioresistant effect through the anti-apoptotic signaling pathway. In experiments using radioresistant lung cancer cells, a knockdown of HSF1 and administration of an HSP90 inhibitor resulted in a high level of cell apoptosis and increased cell sensitivity to radiotherapy [62]. Apart from these positive effects, the inhibition of HSP90 induces the release of HSF1 from the HSP90 complex, thereby stimulating the transcription of the cytoprotective chaperones HSP70 and HSP27 [8][63][64]. Therefore, this forms a feedback loop to counteract the effect of HSP90 inhibition.
5. Targeting HSF1 for Cancer Treatment
Inhibitors of HSF1 are compounds designed to block or modulate the activity of HSF1. These inhibitors have been studied for their potential therapeutic applications, especially in cancer treatment, where HSF1 is often upregulated and contributes to the survival and growth of cancer cells.
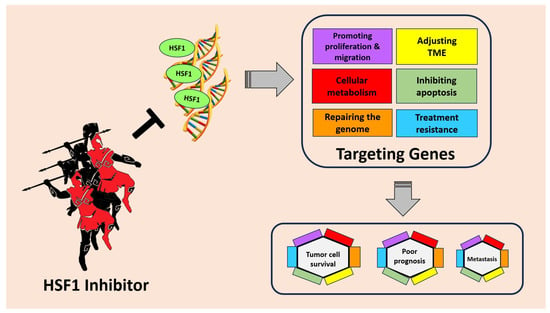
Targeting HSF1 for cancer treatment holds great promise as an innovative therapeutic strategy. HSF1, known for its role in cellular stress response, has emerged as a critical regulator in tumorigenesis. In cancer cells, HSF1 becomes hyperactivated, leading to the overexpression of genes involved in anti-apoptotic mechanisms and cellular metabolism. This heightened HSF1 activity also promotes the migration and invasion of cancer cells, facilitating tumor spread to distant sites. Moreover, HSF1 contributes to drug resistance, making cancer treatments less effective (Figure 3).

Figure 3. HSF1 plays an important role in tumor cell survival, poor prognosis, and metastasis through several mechanisms. HSF1 can increase the expression of genes involved in anti-apoptotic mechanisms, cellular metabolism, promoting migration, and even drug resistance. By inhibiting HSF1, cancer progression can be suppressed and provide better hope to patients.
In light of these findings, scientists have explored inhibiting HSF1 as a potential therapeutic approach. By targeting HSF1, researchers aim to disrupt the pro-cancer effects of this transcription factor. Targeting HSF1 as a cancer therapy is currently in the pre-clinical stage, and it is regarded as a promising cancer treatment strategy. In several malignancies, a decrease in HSF1 activity can inhibit aneuploidy and cancer cell proliferation [64].
Despite successfully blocking HSF1 in vitro and in animal models, each agent has therapeutic limitations. Under stress, HSF1 plays a vital role in cancer and normal cells. Inhibiting HSF1 for anti-cancer treatment can be harmful to normal cells. As a result, it is critical to specifically identify and target cancer cells to reduce cytotoxic effects on normal cells. This necessitates the improvement of existing drugs through synthetic techniques that change functional groups/motifs or the identification and isolation of new natural molecules capable of overcoming possible off-target difficulties [65].
Most current inhibitors indirectly interfere with HSF1, lacking specificity and potency. Developing direct small-molecule inhibitors for HSF1 is challenging due to its complex structure. Moreover, the mechanisms of HSF1 in tumorigenesis and development are complicated, involve diverse signaling pathways, and may depend on different cancer types. Recent advancements in HSF1 drug development have brought renewed hope, exemplified by the discovery of direct HSF1 inhibitors such as DTHIB [29][66] and CCT361814 [67][68][69]. These inhibitors have demonstrated potent and specific suppression of tumor growth in pre-clinical animal studies while displaying low toxicity to normal tissues. Encouragingly, CCT361814 has entered Phase I clinical trials. The prospect of developing new generations of HSF1 inhibitors, especially those directly targeting HSF1 itself, holds promise.
A lack of possible target locations in the tertiary structure makes developing HSF1 inhibitors problematic. HSF-1 is a transcription factor with relatively weak “druggability” [65][70]. Furthermore, its complex activation pathway involves several components, including multichaperone complexes and various PTMs. Nonetheless, promising HSF1 inhibitors have been developed, frequently derived from natural compounds or synthesized chemical structures [46][71]. Below are examples of HSF1 inhibitors tested in vitro and in vivo. So far, NXP800 (CCT361814) is the only HSF1 inhibitor that has entered clinical trials. It is expected that, in the near future, other HSF1 inhibitors will enter clinical trials.
6. Conclusions
HSF1 inhibition has emerged as a possible cancer therapeutic method. HSF1, a protein involved in cellular stress responses, is frequently overexpressed in cancer cells, contributing to tumor growth and resistance to treatment. HSF1 inhibition has been shown in studies to effectively diminish critical cancer features such as cell proliferation, survival, and metastasis. HSF1 inhibitors interfere with protein folding, reducing HSF1’s ability to bind to DNA, disrupting its involvement in gene activation. Notably, blocking HSF1 has been shown to improve the efficacy of traditional chemotherapy, radiation therapy, and targeted treatments, suggesting its compatibility with these standard therapies.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15215167
References
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555.
- Morimoto, R.I. The heat shock response: Systems biology of proteotoxic stress in aging and disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 91–99.
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266.
- Jiang, S.; Tu, K.; Fu, Q.; Schmitt, D.C.; Zhou, L.; Lu, N.; Zhao, Y. Multifaceted roles of HSF1 in cancer. Tumour Biol. 2015, 36, 4923–4931.
- Dai, C.; Sampson, S.B. HSF1: Guardian of Proteostasis in Cancer. Trends Cell Biol. 2016, 26, 17–28.
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017, 27, 895–905.
- Chen, F.; Fan, Y.; Cao, P.; Liu, B.; Hou, J.; Zhang, B.; Tan, K. Pan-Cancer Analysis of the Prognostic and Immunological Role of HSF1: A Potential Target for Survival and Immunotherapy. Oxid. Med. Cell. Longev. 2021, 2021, 5551036.
- Wang, G.; Cao, P.; Fan, Y.; Tan, K. Emerging roles of HSF1 in cancer: Cellular and molecular episodes. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188390.
- Mendillo, M.L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 Drives a Transcriptional Program Distinct from Heat Shock to Support Highly Malignant Human Cancers. Cell 2012, 150, 549–562.
- Wan, T.; Shao, J.; Hu, B.; Liu, G.; Luo, P.; Zhou, Y. Prognostic role of HSF1 overexpression in solid tumors: A pooled analysis of 3,159 patients. Onco Targets Ther. 2018, 11, 383–393.
- Dong, B.; Jaeger, A.M.; Thiele, D.J. Inhibiting Heat Shock Factor 1 in Cancer: A Unique Therapeutic Opportunity. Trends Pharmacol. Sci. 2019, 40, 986–1005.
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19.
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulation of the mammalian heat shock factor 1. FEBS J. 2017, 284, 1606–1627.
- Neudegger, T.; Verghese, J.; Hayer-Hartl, M.; Hartl, F.U.; Bracher, A. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat. Struct. Mol. Biol. 2016, 23, 140–146.
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115.
- Kmiecik, S.W.; Mayer, M.P. Molecular mechanisms of heat shock factor 1 regulation. Trends Biochem. Sci. 2022, 47, 218–234.
- Ravarani, C.N.; Erkina, T.Y.; De Baets, G.; Dudman, D.C.; Erkine, A.M.; Babu, M.M. High-throughput discovery of functional disordered regions: Investigation of transactivation domains. Mol. Syst. Biol. 2018, 14, e8190.
- Pincus, D. Regulation of Hsf1 and the Heat Shock Response. Adv. Exp. Med. Biol. 2020, 1243, 41–50.
- Li, G.; Kryczek, I.; Nam, J.; Li, X.; Li, S.; Li, J.; Wei, S.; Grove, S.; Vatan, L.; Zhou, J.; et al. LIMIT is an immunogenic lncRNA in cancer immunity and immunotherapy. Nat. Cell Biol. 2021, 23, 526–537.
- Masser, A.E.; Ciccarelli, M.; Andreasson, C. Hsf1 on a leash—Controlling the heat shock response by chaperone titration. Exp. Cell Res. 2020, 396, 112246.
- Guettouche, T.; Boellmann, F.; Lane, W.S.; Voellmy, R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005, 6, 4.
- Holmes, B.; Benavides-Serrato, A.; Freeman, R.S.; Landon, K.A.; Bashir, T.; Nishimura, R.N.; Gera, J. mTORC2/AKT/HSF1/HuR constitute a feed-forward loop regulating Rictor expression and tumor growth in glioblastoma. Oncogene 2018, 37, 732–743.
- Srivastava, P.; Takii, R.; Okada, M.; Fujimoto, M.; Nakai, A. MED12 interacts with the heat-shock transcription factor HSF1 and recruits CDK8 to promote the heat-shock response in mammalian cells. FEBS Lett. 2021, 595, 1933–1948.
- Chou, S.D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 2012, 7, e39679.
- Huang, C.Y.; Lee, F.L.; Peng, S.F.; Lin, K.H.; Chen, R.J.; Ho, T.J.; Tsai, F.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. HSF1 phosphorylation by ERK/GSK3 suppresses RNF126 to sustain IGF-IIR expression for hypertension-induced cardiomyocyte hypertrophy. J. Cell. Physiol. 2018, 233, 979–989.
- Raychaudhuri, S.; Loew, C.; Korner, R.; Pinkert, S.; Theis, M.; Hayer-Hartl, M.; Buchholz, F.; Hartl, F.U. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 2014, 156, 975–985.
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066.
- Kmiecik, S.W.; Le Breton, L.; Mayer, M.P. Feedback regulation of heat shock factor 1 (Hsf1) activity by Hsp70-mediated trimer unzipping and dissociation from DNA. EMBO J. 2020, 39, e104096.
- Dong, B.; Jaeger, A.M.; Hughes, P.F.; Loiselle, D.R.; Hauck, J.S.; Fu, Y.; Haystead, T.A.; Huang, J.; Thiele, D.J. Targeting therapy-resistant prostate cancer via a direct inhibitor of the human heat shock transcription factor 1. Sci. Transl. Med. 2020, 12, eabb5647.
- Engerud, H.; Tangen, I.L.; Berg, A.; Kusonmano, K.; Halle, M.K.; Oyan, A.M.; Kalland, K.H.; Stefansson, I.; Trovik, J.; Salvesen, H.B.; et al. High level of HSF1 associates with aggressive endometrial carcinoma and suggests potential for HSP90 inhibitors. Br. J. Cancer 2014, 111, 78–84.
- Fang, F.; Chang, R.; Yang, L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer 2012, 118, 1782–1794.
- Workman, P. Reflections and Outlook on Targeting HSP90, HSP70 and HSF1 in Cancer: A Personal Perspective. Adv. Exp. Med. Biol. 2020, 1243, 163–179.
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320.
- Chin, Y.; Gumilar, K.E.; Li, X.G.; Tjokroprawiro, B.A.; Lu, C.H.; Lu, J.; Zhou, M.; Sobol, W.; Tan, M. Targeting HSF1 for cancer treatment: Mechanisms and inhibitor development. Theranostic 2023, 13, 2281.
- Santagata, S.; Mendillo, M.L.; Tang, Y.C.; Subramanian, A.; Perley, C.C.; Roche, S.P.; Wong, B.; Narayan, R.; Kwon, H.; Koeva, M.; et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 2013, 341, 1238303.
- Yang, T.; Ren, C.; Lu, C.; Qiao, P.; Han, X.; Wang, L.; Wang, D.; Lv, S.; Sun, Y.; Yu, Z. Phosphorylation of HSF1 by PIM2 Induces PD-L1 Expression and Promotes Tumor Growth in Breast Cancer. Cancer Res. 2019, 79, 5233–5244.
- Chang, Z.; Lu, M.; Park, S.M.; Park, H.K.; Kang, H.S.; Pak, Y.; Park, J.S. Functional HSF1 requires aromatic-participant interactions in protecting mouse embryonic fibroblasts against apoptosis via G2 cell cycle arrest. Mol. Cells 2012, 33, 465–470.
- Luft, J.C.; Benjamin, I.J.; Mestril, R.; Dix, D.J. Heat shock factor 1-mediated thermotolerance prevents cell death and results in G2/M cell cycle arrest. Cell Stress Chaperones 2001, 6, 326–336.
- Bruce, J.L.; Chen, C.; Xie, Y.; Zhong, R.; Wang, Y.Q.; Stevenson, M.A.; Calderwood, S.K. Activation of heat shock transcription factor 1 to a DNA binding form during the G(1)phase of the cell cycle. Cell Stress Chaperones 1999, 4, 36–45.
- Fujimoto, M.; Takii, R.; Takaki, E.; Katiyar, A.; Nakato, R.; Shirahige, K.; Nakai, A. The HSF1-PARP13-PARP1 complex facilitates DNA repair and promotes mammary tumorigenesis. Nat. Commun. 2017, 8, 1638.
- Shi, X.; Deng, Z.; Wang, S.; Zhao, S.; Xiao, L.; Zou, J.; Li, T.; Tan, S.; Tan, S.; Xiao, X. Increased HSF1 Promotes Infiltration and Metastasis in Cervical Cancer via Enhancing MTDH-VEGF-C Expression. Onco Targets Ther. 2021, 14, 1305–1315.
- Tian, X.; Zhou, N.; Yuan, J.; Lu, L.; Zhang, Q.; Wei, M.; Zou, Y.; Yuan, L. Heat shock transcription factor 1 regulates exercise-induced myocardial angiogenesis after pressure overload via HIF-1α/VEGF pathway. J. Cell. Mol. Med. 2020, 24, 2178–2188.
- Gabai, V.L.; Meng, L.; Kim, G.; Mills, T.A.; Benjamin, I.J.; Sherman, M.Y. Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol. Cell. Biol. 2012, 32, 929–940.
- Yun, H.H.; Baek, J.Y.; Seo, G.; Kim, Y.S.; Ko, J.H.; Lee, J.H. Effect of BIS depletion on HSF1-dependent transcriptional activation in A549 non-small cell lung cancer cells. Korean J. Physiol. Pharmacol. 2018, 22, 457–465.
- McConnell, J.R.; Buckton, L.K.; McAlpine, S.R. Regulating the master regulator: Controlling heat shock factor 1 as a chemotherapy approach. Bioorg. Med. Chem. Lett. 2015, 25, 3409–3414.
- Mun, G.I.; Choi, E.; Lee, Y.; Lee, Y.S. Decreased expression of FBXW7 by ERK1/2 activation in drug-resistant cancer cells confers transcriptional activation of MDR1 by suppression of ubiquitin degradation of HSF1. Cell Death Dis. 2020, 11, 395.
- Yang, W.; Feng, B.; Meng, Y.; Wang, J.; Geng, B.; Cui, Q.; Zhang, H.; Yang, Y.; Yang, J. FAM3C-YY1 axis is essential for TGFbeta-promoted proliferation and migration of human breast cancer MDA-MB-231 cells via the activation of HSF1. J. Cell. Mol. Med. 2019, 23, 3464–3475.
- Moody, R.; Wilson, K.; Kampan, N.C.; McNally, O.M.; Jobling, T.W.; Jaworowski, A.; Stephens, A.N.; Plebanski, M. Mapping Epitopes Recognised by Autoantibodies Shows Potential for the Diagnosis of High-Grade Serous Ovarian Cancer and Monitoring Response to Therapy for This Malignancy. Cancers 2021, 13, 4201.
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Vydra, N.; Toma, A.; Glowala-Kosinska, M.; Gogler-Piglowska, A.; Widlak, W. Overexpression of heat shock transcription factor 1 enhances the resistance of melanoma cells to doxorubicin and paclitaxe. BMC Cancer 2013, 13, 504.
- Kourtis, N.; Moubarak, R.S.; Aranda-Orgilles, B.; Lui, K.; Aydin, I.T.; Trimarchi, T.; Darvishian, F.; Salvaggio, C.; Zhong, J.; Bhatt, K.; et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat. Cell Biol. 2015, 17, 322–332.
- Schilling, D.; Bayer, C.; Li, W.; Molls, M.; Vaupel, P.; Multhoff, G. Radiosensitization of normoxic and hypoxic h1339 lung tumor cells by heat shock protein 90 inhibition is independent of hypoxia inducible factor-1α. PLoS ONE 2012, 7, e31110.
- Zaidi, S.; McLaughlin, M.; Bhide, S.A.; Eccles, S.A.; Workman, P.; Nutting, C.M.; Huddart, R.A.; Harrington, K.J. The HSP90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage. PLoS ONE 2012, 7, e35436.
- Li, Q.; Martinez, J.D. Loss of HSF1 results in defective radiation-induced G(2) arrest and DNA repair. Radiat. Res. 2011, 176, 17–24.
- Schilling, D.; Kuhnel, A.; Konrad, S.; Tetzlaff, F.; Bayer, C.; Yaglom, J.; Multhoff, G. Sensitizing tumor cells to radiation by targeting the heat shock response. Cancer Lett. 2015, 360, 294–301.
- Jacobs, A.T.; Marnett, L.J. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J. Biol. Chem. 2009, 284, 9176–9183.
- Kim, H.Y.; Kim, Y.-S.; Yun, H.H.; Im, C.-N.; Ko, J.-H.; Lee, J.-H. ERK-mediated phosphorylation of BIS regulates nuclear translocation of HSF1 under oxidative stress. Exp. Mol. Med. 2016, 48, e260.
- Janus, P.; Pakuła-Cis, M.; Kalinowska-Herok, M.; Kashchak, N.; Szołtysek, K.; Pigłowski, W.; Widlak, W.; Kimmel, M.; Widlak, P. NF-κB signaling pathway is inhibited by heat shock independently of active transcription factor HSF1 and increased levels of inducible heat shock proteins. Genes Cells 2011, 16, 1168–1175.
- Li, W.; Hu, C.; Zhong, X.; Wu, J.; Li, G. Melatonin Induces AGS Gastric Cancer Cell Apoptosis via Regulating PERK/eIF2α and HSF1/NF-κB Signaling Pathway. Ann. Clin. Lab. Sci. 2022, 52, 40–47.
- Li, J.; Liu, Y.; Duan, P.; Yu, R.; Gu, Z.; Li, L.; Liu, Z.; Su, L. NF-κB regulates HSF1 and c-Jun activation in heat stress-induced intestinal epithelial cell apoptosis. Mol. Med. Rep. 2018, 17, 3388–3396.
- Kühnel, A.; Schilling, D.; Combs, S.E.; Haller, B.; Schwab, M.; Multhoff, G. Radiosensitization of HSF-1 Knockdown Lung Cancer Cells by Low Concentrations of Hsp90 Inhibitor NVP-AUY922. Cells 2019, 8, 1166.
- Carpenter, R.L.; Gokmen-Polar, Y. HSF1 as a Cancer Biomarker and Therapeutic Target. Curr. Cancer Drug Targets 2019, 19, 515–524.
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007, 130, 1005–1018.
- Nytko, K.J.; Thumser-Henner, P.; Russo, G.; Weyland, M.S.; Rohrer Bley, C. Role of HSP70 in response to (thermo)radiotherapy: Analysis of gene expression in canine osteosarcoma cells by RNA-seq. Sci. Rep. 2020, 10, 12779.
- Dong, Q.; Xiu, Y.; Wang, Y.; Hodgson, C.; Borcherding, N.; Jordan, C.; Buchanan, J.; Taylor, E.; Wagner, B.; Leidinger, M.; et al. HSF1 is a driver of leukemia stem cell self-renewal in acute myeloid leukemia. Nat. Commun. 2022, 13, 6107.
- Workman, P.; Clarke, P.A.; Te Poele, R.; Powers, M.; Box, G.; De Billy, E.; De Haven Brandon, A.; Hallsworth, A.; Hayes, A.; McCann, H.; et al. Discovery and validation of biomarkers to support clinical development of NXP800: A first-in-class orally active, small-molecule HSF1 pathway inhibitor. Eur. J. Cancer 2022, 174, S35.
- Menezes, K.; Aram, G.; Mirabella, F.; Johnson, D.C.; Sherborne, A.L.; Houlston, R.S.; Cheeseman, M.D.; Pasqua, E.; Clarke, P.; Workman, P.; et al. The Novel Protein HSF1 Stress Pathway Inhibitor Bisamide CCT361814 Demonstrates Pre-Clinical Anti-Tumor Activity in Myeloma. Blood 2017, 130, 3072.
- Diane Marsolini, S.S. A Phase 1 Clinical Study of NXP800 in Subjects With Advanced Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT05226507?term=HSF1&draw=2&rank=3#contacts (accessed on 2 September 2023).
- Cheeseman, M.D.; Chessum, N.E.; Rye, C.S.; Pasqua, A.E.; Tucker, M.J.; Wilding, B.; Evans, L.E.; Lepri, S.; Richards, M.; Sharp, S.Y.; et al. Discovery of a Chemical Probe Bisamide (CCT251236): An Orally Bioavailable Efficacious Pirin Ligand from a Heat Shock Transcription Factor 1 (HSF1) Phenotypic Screen. J. Med. Chem. 2017, 60, 180–201.
- Zhang, D.; Zhang, B. Selective killing of cancer cells by small molecules targeting heat shock stress response. Biochem. Biophys. Res. Commun. 2016, 478, 1509–1514.
- Kim, J.A.; Kim, Y.; Kwon, B.M.; Han, D.C. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 2013, 288, 28713–28726.
This entry is offline, you can click here to edit this entry!
