Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Traditional meat products are commonly produced in small family businesses. However, big industries are also involved in the production of this kind of product, especially since a growing number of consumers crave the traditional taste and aromas. The popularization of original and organic products has resulted in a return to traditional production methods. Traditional meat products are produced worldwide. However, in such (domesticated) conditions there is a potential danger for mycotoxin contamination.
- dry sausage
- cured meat products
- mycotoxins
- traditional production
- fungi
1. Introduction
Traditional meat products mostly refers to dry-cured meat products produced in a traditional manner. The majority of meat industries try to imitate the traditional production, but such products always show a certain discrepancy in aroma and texture when compared to traditionally produced cured meat products. However, the demand for high-quality and health-safe products enables many small producers to step in the market with traditional meat products. Different dry-cured meat products require high quality meat and production conditions that satisfy basic health and hygienic standards [1].
2. Traditional Dry-Cured Products—Common Fungi
Traditional dry-cured meat products are indigenous to many countries, especially in Europe where molds play an important role in the ripening stage of production (Figure 1). Italy, Spain, France, Hungary, Croatia, and Southern Germany traditionally use white and occasionally green mold cover on the surface of dry-cured products. They are greatly appreciated due to the development of a characteristic taste, flavor, texture, and appearance of dry-cured meat products. The color of the mold cover depends on the species, but often it is influenced by the temperature during the ripening phase. For example, growth above 15 °C stimulated green conidia formation [2]. This is particular for traditional manufacture since they usually do not have controlled humidity and temperature conditions and depend on environmental conditions, thus the resulting molds can sometimes be white and sometimes greenish. Common molds used in some traditional dry-cured meat products are filamentous fungi such as Alternaria, Aspergillus, Cladosporium, Eurotium, Mucor, Penicillium, Rhizopus, and Scopulariopsis, as reported in the studies focused on fermented sausages and dry-cured hams [3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31], as shown in Figure 2. Penicillium was detected and identified in some samples of traditionally produced fermented sausages and only in several samples of dry-cured ham [3][5][12][15][19][32]. In some samples it was identified together with Scopulariopsis [9], Aspergillus [4][8], and Eurotium [33].
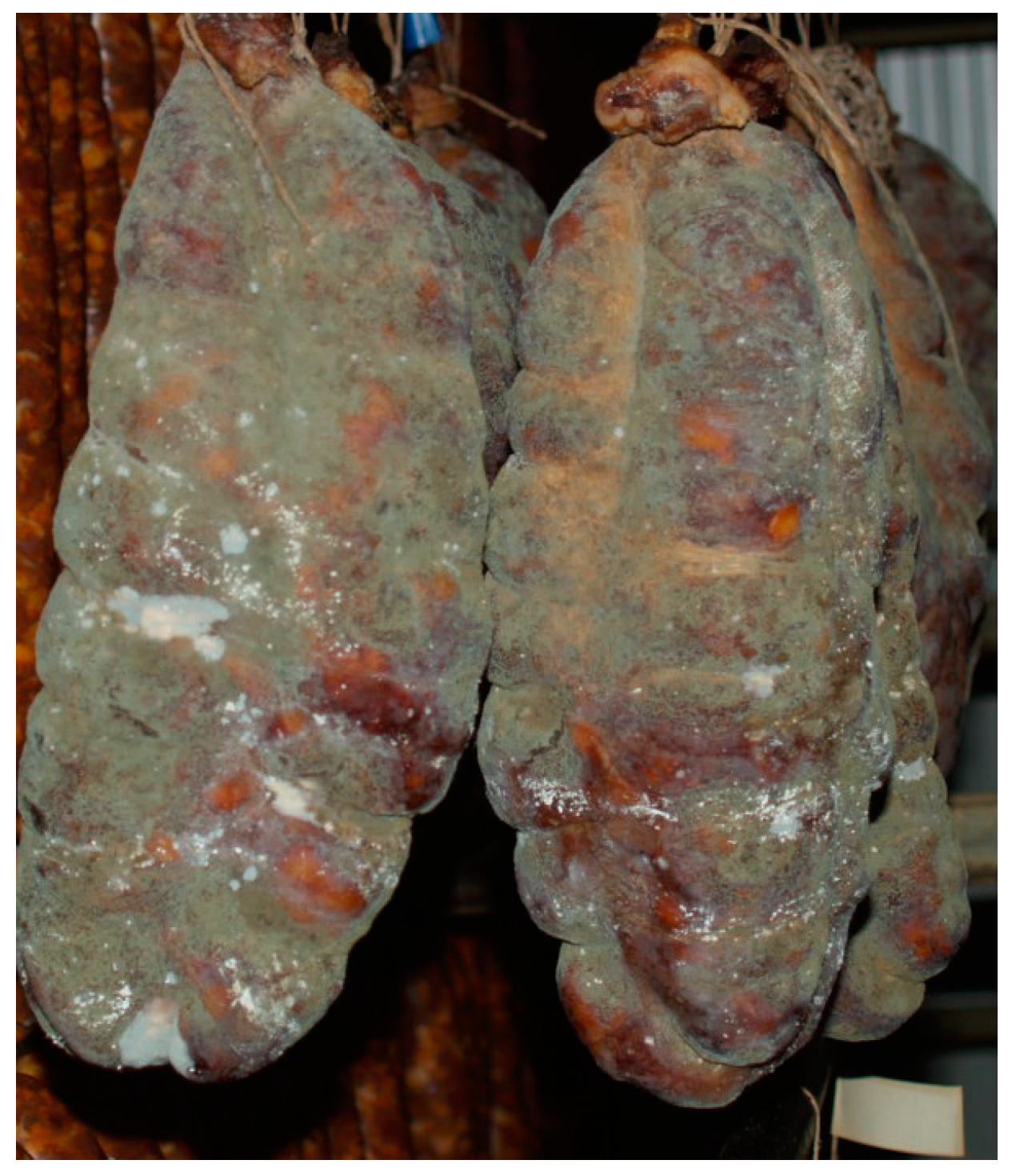
Figure 1. Molds on the traditional Croatian dry-cured sausage Slavonski Kulen.
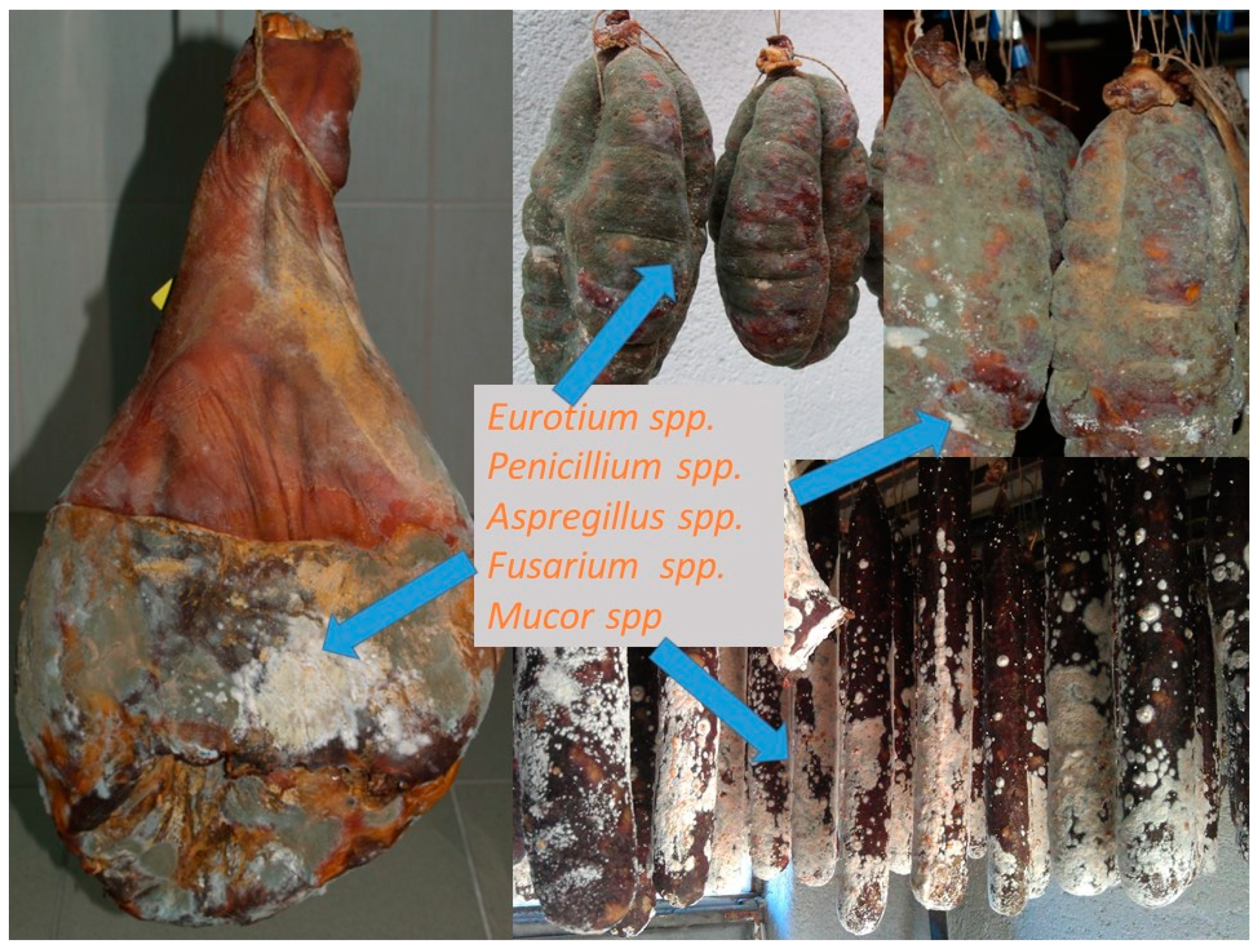
Figure 2. Different molds on traditional dry sausages and dry-cured meat products.
According to several sources, Aspergillus and Eurotium have been shown to be the prevailing molds on dry-cured hams [12][14][16][29][30][32]. Molds belonging to genera Eurotium are xerophilic and prosper on substrates such as dry-cured hams, with surfaces with low water activity (<0.80) [20]. Fermented sausages were commonly contaminated with Penicillium spp. Similarly to Eurotium spp., Penicillium species tolerate low water activity (0.78–0.83) and protein-rich substrates. They proliferate in lower to mid temperatures [30][31][34]. Some species belonging to Penicillium genera, such as Penicillium nalgiovense have been used as mold starter cultures for the industrial production of mold-fermented sausage [2][31]. Penicillium nordicum, as an ochratoxin A producing species, was shown to have a significant share in the studies where it was reported [32][33].
The most common fungi and mycotoxins detected on different meat products are shown in Table 1. As can be seen, different fungal species can be found on meat products. Various mycotoxins can be produced by fungi, but some can be found in combination, which is called co-occurrence. This is an especially health-concerning topic for scholars since multiple toxins can be detrimental to human health with a combination of side-effects.
Table 1. Most common fungi and mycotoxins detected on different meat products (adopted from [35]).
| Fungi | Mycotoxin |
|---|---|
| Aspergillus spp. | |
| A. flavus | Aflatoxin B1, cyclopiazonic acid, 3-nitropropionic acid |
| A. niger | Ochratoxin A, fumonisin B2 |
| A. ochraceus | Ochratoxin A, penicillic acid, xanthomegnin, viomellein, vioxanthin |
| A. versicolor | Sterigmatocystin |
| Penicillium spp. | |
| P. aurantiogriseum | Penicillic acid, verrucosidin, terrestric acid, nephrotoxic glycopeptides |
| P. brevicompactum | Botryodiploidin |
| P. chrysogenum | Secalonic acid, PR toxin, roquefortine C |
| P. citrinum | Citrinin |
| P. commune | Cyclopiazonic acid |
| P. crustosum | Terrestric acid, penitrems, roquefortine C |
| P. expansum | Patulin, citrinin, chaetoglobosins, communesins, roquefortine C |
| P. glabrum | Citromycetin |
| P. griseofulvum | Patulin, griseofulvins, roquefortine C, cyclopiazonic acid |
| P. nordicum | Ochratoxin A, viridic acid |
| P. oxalicum | Secalonic acids, roquefortine C |
| P. palitans | Cyclopiazonic acid |
| P. roquefortii | PR toxin, roquefortine C |
| P. rugulosum | Rugulosin |
| P. variabile | Rugulosin |
| P. verrucosum | Ochratoxin A, citrinin |
| P. viridicatum | Penicillic acid, xanthoemegnins, viridic acid |
Environmental contamination of meat products can occur via conidia, ascospores, or mycelium fragments, but not many of them can grow on meat products, especially dry-cured ones [30]. According to [17] Cladosporium was found to be the dominating contaminant in the fermented sausages.
3. Mycotoxins in Dry Sausages and Dry-Cured Meat Products
Mycotoxins can end up in dry-cured products through different pathways. Most commonly they end up in animals via contaminated feed. The addition of spices in the meat and/or stuffing can also contribute to the contamination. However, many scientific investigations have aimed to clarify the origin of mycotoxins in dry-cured meat products and relied on the thesis that molds growing on the surface of the meat product can be the source of contamination. Even though EU legislation does not yet include mycotoxins in meat and meat products, OTA has currently been under consideration by the EFSA’s scientific opinions. More will be described in the following sections.
Mycotoxins enter the food supply chain and end up in markets which can be detrimental to human health. There are two ways for mycotoxins to enter the food and feed chain: direct and indirect [36][37]. Meat as a raw material can be contaminated via the feed used for animal feeding (carry-over effects), and consequently, can indirectly contribute to human exposure. In addition, spices used for meat products’ production are reportedly a potential direct source of mycotoxins [38][39]. Comi and Iacumin [40] reported OTA in hams, probably as a result of direct contamination with molds. This can also be explained by indirect contamination as described by several authors [41][42][43]. Similarly, OTA can be found in sausages. The pathway of contamination can be identified as indirect transmission via pigs’ feed and by direct contamination via molds which can grow on raw meat after slaughter [44][45]. The environment in which the production is carried out can also be a source of fungi that can proliferate in meat products during the ripening phase. This phase is particularly important since it involves high humidity and favorable temperatures for fungal growth.
The production of traditional meat products, especially dry sausages, relies on the addition of different spices to deliver the familiar aromas and taste. The most used spices are pepper (white, red, and black), sweet and spicy ground paprika, and garlic. Certain traditional products can be spiced with laurel and rosemary [46]. Despite the antifungal activity that many of these spices display, they can be contaminated with molds as well [47]. Common contaminants found in spices belong to Aspergillus and Penicillium genera [48][49][50][51][52]. Due to poor production conditions (drying spices on the ground) spices such as chili, nutmeg, and paprika powders can contain aflatoxins (AFs), OTA, and certain different mycotoxins. Some samples even exceed the maximum EU legislative limits. Spices sold at farmers markets can even have significantly higher mycotoxin concentrations than those bought at supermarkets [46][53][54]. Spices such as paprika and black pepper can contain significant amounts of AFs and OTA [55][56][57][58][59]. According to Gambacorta et al. [55], AFB1 concentrations in paprika can reach 155.7 µg/kg, and in black pepper it can be up to 75.8 µg/kg [56]. High values of OTA can also be found in paprika, amounting to 177.4 µg/kg [55] and 79.0 µg/kg in black pepper [57]. A higher prevalence of OTA in prosciutto samples was sometimes linked to pepper spiking; namely, pepper often becomes contaminated with Aspergillus molds, out of which A. niger produces OTA [8][50]. However, some studies have revealed that spices may also inhibit mold growth [51], resulting, for instance, in lower OTA contamination in some meat products [8].
OTA can also be found in certain prosciutto samples due to pepper used for coating, since molds such as Aspergillus spp. commonly contaminate pepper [60]. OTA can be found in dry-cured hams, as well. However, according to the literature, this contamination probably occurs during ripening, due to direct contamination with molds, presumably because of the inadequate environmental conditions, i.e., increased air humidity and higher temperatures [32][61][62][63].
Sometimes, damages to the outer casing can enable the entrance of molds and mycotoxins into sausages (stuffing) (Figure 3), and in significant amounts, according to [60][63][64].

Figure 3. Molds inside a dry sausage due to damaged casing.
However, Iacumin et al. [33] reported OTA contamination of the casings (outer layer) while the inner layer was not contaminated. According to [65] this can also present health hazard since casings are usually sliced together and consumed with the stuffing.
OTA is a natural contaminant in different foodstuff and is often detected in meat products. Aspergillus ochraceus and Penicillium verrucosum are the most common producers of OTA concerning the meat industry. Direct contamination usually occurs via animal feed [66][67][68]. OTA is fat-soluble and can mostly be found in the kidney, lung, liver, blood, spleen, heart, and adipose tissue of pigs [68][69]. A study conducted by Perši et al. [38] showed that pigs who were given 300 μg/kg/day of OTA for 30 days, accumulated it in the kidneys, lungs, and fat tissue. OTA was then detected in different meat products such as blood sausages, liver sausage, and pâté [70]. Even though OTA is not legally regulated in the EU, some countries have recognized the importance of strict limits for this mycotoxin and have set the limit to 5 μg/kg in pig liver, kidneys, and meat (Romania). In Italy the limit is much lower, set at 1 μg/kg in pig meat and meat products [70]. Suppression of OTA in meat products can be carried out by proper prevention via food safety management systems. In cases where prevention is not sufficient, then a set of diverse methods of physical and chemical treatments can be applied in order to reduce the contamination. However, chemicals used for decontamination can impair the sensory properties of dry-cured products which can drive the customers away. Much research has been devoted to finding a biological prevention method, and so far, essential oils have been meticulously investigated for this purpose. Even though essential oils can significantly affect the sensory properties of such products, the involvement of novel encapsulation technologies could help reduce such changes [71]. So far, oregano, garlic, sage, peppermint, rosemary, neem, and eucalyptus have been identified as being successful in suppressing mold growth and OTA production [72][73][74].
Aflatoxins are commonly produced by Aspergillus spp. (A. flavus and A. parasiticus). Aflatoxin B1 (AFB1) is known for its high potential for carcinogenic and genotoxic properties. Its metabolite is AFM1, an aflatoxin that can be found in milk, where it ends up through ingestion of contaminated feed. AFB1 is not often determined in meat foods, and when it is, its concentrations are much lower than OTA. It can also be found in different tissues such as liver, muscle, and fat tissue [75]. According to the IARC (International Agency for Research on Cancer) it belongs to Group 1 (human carcinogen) and is associated with the occurrence of liver cancer [76][77]. Even though AFB1 is not as common as OTA, it can still be found in different processed meat products [78]. Similarly, like OTA, AFs can be efficiently suppressed by using essential oils. As an effective agent, onion has been designated as an effective AF inhibitor in the meat industry [79]. Saffron, Shirazi thyme, estragon, basil, black cumin, coriander, dill seeds, and Arabian incense can suppress AF production [80][81][82][83][84][85][86][87][88].
Zearalenone (ZEA) is designated as the oestrogenic mycotoxin, according to the IARC it is described as a Group 3 carcinogen. ZEA causes hormonal disbalance related to cervical, ovarian, and prostate cancer [89][90]. Its derivate, α-zeranol, can be used as a cattle growth agent, but so far, the EU has not approved this [71]. ZEA is commonly found in chicken meat [91], sheep meat, and beef meat [92]. To reduce ZEA levels lemon, grapefruit, eucalyptus, and palmarosa essential oils were investigated, but the activity of these oils cannot be interpreted as being significantly effective [93].
According to some authors, citrinin (CIT) can be found in dry-cured traditional meat products as well [94]. Citrinin displays hepatic and nephrotoxic effects, and is generally produced by Penicillium spp., but specifically by Penicillium citrinum. Aspergillus, and Monascus genera can also synthesize this mycotoxin, originally named monascidin. CIT can be found in kidneys, causing renal degeneration associated with weight loss [95][96]. According to the IARC it belongs to Group 3 [97]. Even though it can be found in different meats and meat products including dry-cured meat products [94][98][99], there is a minimal contribution to increased CIT intake in humans, given the low rate of CIT transfer from feed to tissue for consumption [100][101].
Patulin (PAT) is mycotoxin synthesized by several species belonging to genera Penicillium, Aspergillus, and Byssochlamys. It displays toxigenic properties [102]. PAT has carcinogenic potential, being classified as being in Group 3 by the IARC as well [103]. In meat products PAT usually co-appears with other mycotoxins. PAT and OTA were detected in dry-cured hams [104]. Since PAT is not incidental, very few studies have been conducted regarding the usage of essential oils in patulin suppression in meat products [71].
Sterigmatocystin (STC) is in Group 2B, according to the IARC [105]. It can be found in pork muscle [106].
Fusarenon-X (FX) is designated as a trichothecene belonging to group B, and the IARC classified it as Group 3. It can often be found in food and feed. In livers and kidneys, it can be converted to nivalenol. The IARC has classified these toxins as belonging to Group 3 [107][108].
T-2 toxin is often found in cereals and cereal based products and is a metabolic product from Fusarium, Myrothecium, and Stachybotrys genera [109]. T-2 was detected in back muscle, pig back fat, and chicken muscle in concentrations less than 0.5 μg/kg [69].
Deoxynivalenol (DON) causes acute emesis, gastroenteritis, diarrhea, and reduced food consumption with chronic implications. It can be found in pig back fat, muscles, and liver [110][111].
Cyclopiazonic acid (CPA) is characterized as a dangerous mycotoxin that can cause damage to the digestive organs, the myocardium, and the skeletal muscles, and cause neurological disorders. Its producers belong to Penicillium and Aspergillus spp., specifically Penicillium commune [21][22][23] which was isolated from the surfaces of different meat products, including European dry-fermented sausages and prosciuttos [5][12][24][112][113][114].
This entry is adapted from the peer-reviewed paper 10.3390/life13112211
References
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Langsrud, S.; Omer, M.K.; Nesbakken, T.; Skaar, I. A HACCP plan for mycotoxigenic hazards associated with dry-cured meat production processes. Food Control 2011, 22, 831–837.
- Sunesen, L.O.; Stahnke, L.H. Mould starter cultures for dry sausages—Selection, application and effects. Meat Sci. 2003, 65, 935–948.
- Mizáková, A.; Pipová, M.; Turek, P. The occurence of moulds in fermented raw meat products. Czech J. Food Sci. 2002, 20, 89–94.
- Strzelecki, E.L.; Badura, L. Occurrence of Aflatoxinogenic Molds on Dry Cracower Sausage. Acta Microbiol. Pol. Ser. B-Microbiol. Appl. 1972, 4, 233–239.
- Andersen, S.J. Compositional Changes in Surface Mycoflora during Ripening of Naturally Fermented Sausages. J. Food Protec. 1995, 58, 426–429.
- Dragoni, I.; Cantoni, C.; Papa, A. Microflora of Carnian dry sausages. Ind. Aliment. 1991, 30, 842–844.
- Feofilova, E.P.; Kuznetsova, L.S.; Sergeeva, Y.E.; Galanina, L.A. Species composition of food-spoiling mycelial fungi. Microbiology 2009, 78, 112–116.
- Grazia, L.; Romano, P.; Bagni, A.; Roggiani, D.; Guglielmi, G. The role of moulds in the ripening process of salami. Food Microbiol. 1986, 3, 19–25.
- Jircovsky, M.; Galgóczy, J. Investigations into the mould flora of Hungarian Winter salami. Die Fleischwirtsch. 1966, 46, 128.
- Leistner, L.; Ayres, J.C. Mold fungi and meat products. Die Fleischwirtsch. 1967, 47, 1320–1326.
- Leistner, L.; Eckardt, C. Occurence of toxinogenic Penicillia in meat products. Die Fleischwirtsch. 1979, 59, 1892–1896.
- Lopez-Diaz, T.M.; Santos, J.A.; Garcia-Lopez, M.L.; Otero, A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001, 68, 69–74.
- Matos, T.J.S.; Jensen, B.B.; Bernardo, F.M.A.; Barreto, A.H.S.; Hojberg, O. Mycoflora of two types of Portuguese dry-smoked sausages and inhibitory effect of sodium benzoate, potassium sorbate, and methyl p-hydroxybenzoate on mold growth rate. J. Food Prot. 2007, 70, 1468–1474.
- Mutti, P.; Previdi, M.P.; Quintavalla, S.; Spotti, E. Toxigenity of mould strains isolated from salami as a function of culture medium. Ind. Conserve 1988, 63, 142–145.
- Papagianni, M.; Ambrosiadis, I.; Filiousis, G. Mould growth on traditional Greek sausages and penicillin production by Penicillium isolates. Meat Sci. 2007, 76, 653–657.
- Racovita, A.; Racovita, A.; Constantinescu, T. The importance of mould layers on salami. Die Fleischwirtsch. 1969, 49, 461–466.
- Skrinjar, M.; Horvar-Skenderovic, T. Contamination of dry sausage with moulds, aflatoxin, achratoxin and zearalenone. Tehnol. Mesa 1989, 30, 53–59.
- Tabuc, C.; Bailly, J.D.; Bailly, S.; Querin, A.; Guerre, P. Toxigenic potential of fungal mycoflora isolated from dry cured meat products: Preliminary study. Rev. Med. Vet. 2004, 155, 287–291.
- Asefa, D.T.; Gjerde, R.O.; Sidhu, M.S.; Langsrud, S.; Kure, C.F.; Nesbakken, T.; Skaar, I. Moulds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009, 128, 435–439.
- Casado, M.K.; Borrás, M.D.; Aguilar, R.V. Fungal flora present on the surface of cured Spanish ham. Die Fleischwirtsch. 1991, 71, 1300–1302.
- Comi, G.; Orlic, S.; Redzepovic, S.; Urso, R.; Iacumin, L. Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int. J. Food Microbiol. 2004, 96, 29–34.
- Dragoni, I.; Marino, C.; Cantoni, C. “Bresaole” and raw hams surface moulds. Ind. Aliment. 1980, 19, 405–407.
- Monte, E.; Villanueva, J.R.; Domínquez, A. Fungal profiles of Spanish country-cured hams. Int. J. Food Microbiol. 1986, 3, 355–359.
- Peintner, U.; Geiger, J.; Pöder, R. The Mycobiota of Speck, a Traditional Tyrolean Smoked and Cured Ham. J. Food Protec. 2000, 63, 1399–1403.
- Spotti, E.; Mutti, P.; Campanini, M. Occurence of moulds on hams during preripening and ripening: Contamination of the environment and growth on the muscle portion of hams. Ind. Conserve 1989, 64, 110–113.
- Spotti, E.; Chiavaro, E.; Lepiani, A.; Colla, F. Mould and ochratoxin A contamination of pre-ripened and fully ripened hams. Ind. Conserve 2001, 76, 341–354.
- Sutic, M.; Ayres, J.C.; Koehler, P.E. Identification and Aflatoxin Production of Molds Isolated from Country Cured Hams. Appl. Microbiol. 1972, 23, 656–658.
- Rojas, F.J.; Jodral, M.; Gosalvez, F.; Pozo, R. Mycoflora and toxinogenic Aspergillus flavus in Spanish dry-cured ham. Int. J. Food Microbiol. 1991, 13, 249–256.
- Huerta, T.; Sanchis, V.; Hernandez, J.; Hernandez, E. Mycoflora of dry-salted Spanish ham. Microbiol. Aliment. Nutr. 1987, 5, 247–252.
- Filtenborg, O.; Frisvad, J.C.; Samson, R.A. Specific association of fungi to foods and influence of physical environmental factors. In Introduction to Food- and Airborne Fungi, 6th ed.; Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., Eds.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; pp. 306–320.
- Leistner, L.; Eckardt, C. Schimmelpilze und Mykotoxine in Fleisch und Fleischerzeugnissen. In Mykotoxine in Lebensmitteln; Reiss, J., Ed.; Gustav Fisher Verlag: Stuttgartt, Germany, 1981; pp. 297–341.
- Battilani, P.; Pietri, V.A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007, 70, 975–980.
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orli´c, S.; Comi, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70.
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–173.
- Sørensen, L.M.; Frisvad, J.C.; Nielsen, P.V.; Lametsch, R.; Koch, A.G.; Jacobsen, T. Filamentous Fungi on Meat Products, Their Ability to Produce Mycotoxins and a Proteome Approach to Study Mycotoxin Production; Technical University of Denmark (DTU): Kongens Lyngby, Denmark, 2009.
- Pizzolato Montanha, F.; Anater, A.; Burchard, J.F.; Luciano, F.B.; Meca, G.; Manyes, L.; Pimpão, C.T. Mycotoxins in dry-cured meats: A review. Food Chem. Toxicol. 2018, 111, 494–502.
- Rocha, M.E.B.; Freire, F.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165.
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA treated pigs. Meat Sci. 2014, 96, 203–210.
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33.
- Comi, G.; Iacumin, L. Ecology of moulds during the pre-ripening and ripening of San Daniele dry cured ham. Food Res. Int. 2013, 54, 1113–1119.
- Rodríguez, A.; Medina, A.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119.
- Bertuzzi, T.; Gualla, A.; Morlacchini, M.; Pietri, A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control 2013, 34, 79–83.
- Lippolis, V.; Ferrara, M.; Cervellieri, S.; Damascelli, A.; Epifani, F.; Pascale, M.; Perrone, G. Rapid prediction of ochratoxin A-producing strains of Penicillium on dry-cured meat by MOS-based electronic nose. Int. J. Food Microbiol. 2016, 218, 71–77.
- Lusky, K.; Tesch, R.; Gobel, R. The effect of natural and crystalline ochratoxin A in pigs after 28 day-feeding period and the residues of the mycotoxin in the body fluids organs and meat products. Arch. Lebensmittelhyg. 1995, 46, 45–48.
- Iacumin, L.; Milesi, S.; Pirani, S.; Comi, G.; Chiesa, L.M. Ochratoxigenic mold and ochratoxin a in fermented sausages from different areas in northern Italy: Occurrence, reduction or prevention with ozonated air. J. Food Saf. 2011, 31, 538–545.
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F. Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789.
- Hamad, S.H. Factors Affecting the Growth of Microorganisms in Food. In Progress in Food Preservation; Bhat, R., Alias, A.K., Paliyath, G., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 405–427.
- Mandeel, Q.A. Fungal contamination of some imported spices. Mycopathologia 2005, 159, 291–298.
- Farghaly, R.M. Occurrence and significance of moulds and their mycotoxins in spices as meat additives. Ben. Vet. Med. J. 2006, 17, 35–46.
- Bokhari, F.M. Spices mycobiota and mycotoxins available in Saudi Arabia and their abilities to inhibit growth of some toxigenic fungi. Myco J. 2007, 35, 47–53.
- Kocić-Tanackov, S.D.; Dimić, G.R.; Karalic, D. Contamination of spices with moulds potential producers of sterigmatocystine. Acta Period. Technol. 2007, 38, 29–35.
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin producing fungi. Saudi Biol. Sci. J. 2010, 17, 167–175.
- Martins, M.L.; Martins, H.M.; Bernardo, F. Aflatoxins in spices marketed in Portugal. Food Addit. Contam. 2001, 18, 315–319.
- Jalili, M.; Jinap, S. Natural occurrence of aflatoxins and ochratoxin A in commercial dried chili. Food Control 2012, 24, 160–164.
- Gambacorta, L.; Magistà, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotox J. 2018, 11, 159–174.
- Zahra, N.; Khan, M.; Mehmood, Z.; Saeed, M.; Kalim, I.; Ahmad, I.; Malik, K. Determination of aflatoxins in spices and dried fruits. J. Sci. Res. 2018, 10, 315–321.
- Jacxsens, L.; Yogendrarajaha, P.; Meulenaer, B. Risk assessment of mycotoxins and predictive mycology in Sri Lankan spices: Chilli and pepper. Procedia Food Sci. 2016, 6, 326–330.
- Pleadin, J.; Kovačević, D.; Perši, N. Ochratoxin A contamination of the autochthonous dry-cured meat product “Slavonski Kulen” during a six-month production process. Food Control 2015, 57, 377–384.
- Karan, D.D.; Vukojević, J.B.; Ljaljević-Grbić, M.V.; Miličević, D.R.; Janković, V.V. Presence of moulds and mycotoxins in spices. Proc. Nat. Sci. Matica Srp. 2005, 108, 77–84.
- Pleadin, J.; Malenica Staver, M.; Vahčić, N.; Kovačević, D.; Milone, S.; Saftić, L.; Scortichini, G. Survey of aflatoxin B1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77.
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F.; Tantillo, M.G. Ochratoxin A determination in paired kidneys and muscle samples from swines slaughtered in southern Italy. Food Control 2006, 17, 114–117.
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from Northern Italy. Ital. J. Food Sci. 2006, 18, 99–106.
- Lusky, K.; Tesch, D.; Gobel, R. Influence of the mycotoxin ochratoxin A on animal health and formation of residues in pigs and different types of sausages derived from these animals. Arch. Lebensmittelhyg. 1993, 44, 131–134.
- Pleadin, J.; Kovačević, D.; Perković, I. Impact of casing damaging on aflatoxin B1 concentration during the ripening of dryfermented sausages. J. Immunoass. Immunochem. 2015, 36, 655–666.
- Roncada, P.; Altafini, A.; Fedrizzi, G.; Guerrini, A.; Polonini, G.L.; Caprai, E. Ochratoxin A contamination of the casing and the edible portion of artisan salamis produced in two Italian regions. World Mycotoxin J. 2020, 13, 553–562.
- Magan, N.; Olsen, M. (Eds.) Mycotoxins in Food: Detection and Control; Woodhead Publishing: Abington, MA, USA, 2004.
- Bui-Klimke, T.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2014, 55, 1860–1869.
- Tolosa, J.; Ruiz, M.J.; Ferrer, E.; Vila-Donat, P. Ochratoxin A: Occurrence and carry-over in meat a nd meat by-products. A Review. Toxicology 2020, 37, 106–110.
- Bhat, R.; Rai, R.; Karim, A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81.
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259.
- Gheorghe-Irimia, R.A.; Tăpăloagă, D.; Tăpăloagă, P.R.; Ilie, L.I.; Șonea, C.; Serban, A.I. Mycotoxins and Essential Oils—From a Meat Industry Hazard to a Possible Solution: A Brief Review. Foods 2022, 11, 3666.
- Gai, F.; Pattono, D. Ochratoxin A (OTA) Occurence in Meat and Dairy Products: Prevention and Remediation Strategies; Nova Sience Publisher: New York, NY, USA, 2020; pp. 1–31.
- Koteswara, V.; Girisham, S.; Reddy, S. Inhibitory effect of essential oils on growth and ochratoxin a production by Penicillium species. Res. J. Microbiol. 2015, 10, 222–229.
- Álvarez, M.; Delgado, J.; Núñez, F.; Roncero, E.; Andrade, M. Proteomic approach to unveil the ochratoxin A repression by Debaryomyces hansenii and rosemary on Penicillium nordicum during dry-cured fermented sausages ripening. Food Control 2022, 137, 108695.
- Pleadin, J.; Lešić, T.; Milićević, D.; Markov, K.; Šarkanj, B.; Vahčić, N.; Kmetič, I.; Zadravec, M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes 2021, 9, 2122.
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1987; Volume 1–42, pp. 83–87.
- International Agency for Research on Cancer (IARC). Aflatoxins. In Chemical Agents and Related Occupations: A Review of Human Carcinogens; IARC Press: Lyon, France, 2012; Volume 100F, pp. 225–248.
- Elzupir, A.; Abdulkhair, B. Health risk from aflatoxins in processed meat products in Riyadh, KSA. Toxicon 2020, 181, 1–5.
- Zohri, A.; Abdel-Gawad, K.; Saber, S. Antibacterial, antidermatophytic and antitoxigenic activities of onion (Allium cepa L.) oil. Microbiol. Res. 1995, 150, 167–172.
- Tzanidi, C.; Proestos, C.; Markaki, P. Saffron (Crocus sativus L.) inhibits aflatoxin B1 production by Aspergillus parasiticus. J. Adv. Microbiol. 2012, 2, 310–316.
- Fakour, M.; Alameh, A.; Rasouli, I.; Mazaheri, M. Antifungal effects of Zataria multiflora Boiss. and Thymus eriocalyx (ronniger) Jalas essential oils on aflatoxin producing Aspergillus parasiticus. Iran. J. Med. Aromat. Plants 2007, 23, 269–277.
- Alinezhad, S.; Kamalzadeh, A.; Rezaee, M.; Jaimand, K.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Inhibitory effects of some native medicinal plants on Aspergillus parasiticus growth and aflatoxin production. Acta Hortic. 2012, 963, 207–210.
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N. Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) Sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea seeds. Food Control 2012, 25, 27–33.
- Atanda, O.; Akpan, I.; Oluwafemi, F. The potential of some spice essential oils in the control of A. parasiticus CFR 223 and aflatoxin production. Food Control 2007, 18, 601–607.
- Abdel-Wahhab, M.; Aly, S. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J. Appl. Toxicol. 2005, 25, 218–223.
- Abou El-Soud, N.; Deabes, M.; Abou El-Kassem, L.; Khalil, M. Antifungal activity of family Apiaceae essential oils. J. Appl. Sci. Res. 2012, 8, 4964–4973.
- Prakash, B.; Singh, P.; Kedia, A.; Dwivedy, A.; Singh, A.; Dubey, N. Mycoflora and aflatoxin analysis of Arachis hypogaeal and assessment of Anethum graveolensl seed and leaf essential oils against isolated fungi, aflatoxin production and their antioxidant activity. J. Food Saf. 2012, 32, 481–491.
- El-Nagerabi, S.; Elshafie, A.; AlKhanjari, S.; Al-Bahry, S.; Elamin, M. Biological activities of Boswellia sacra extracts on the growth and aflatoxins secretion of two aflatoxigenic species of Aspergillus species. Food Control 2013, 34, 763–769.
- Chang, H.; Kim, W.; Park, J.-H.; Kim, D.; Kim, C.-R.; Chung, S.; Lee, C. The Occurrence of Zearalenone in South Korean Feedstuffs between 2009 and 2016. Toxins 2017, 9, 223.
- WHO/IARC: Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. P R E A M B L E. 2006. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/CurrentPreamble.pdf (accessed on 1 August 2023).
- Mirocha, C.; Robison, T.; Pawlosky, R.; Allen, N. Distribution and residue determination of zearalenone in broilers. Toxicol. Appl. Pharmacol. 1982, 66, 77–87.
- Jonker, M.; van Egmond, H.; Stephany, R. Mycotoxins in Food of Animal Origin: A Review. Available online: https://www.rivm.nl/bibliotheek/digitaaldepot/389002_095.pdf (accessed on 13 July 2023).
- Velluti, A.; Sanchis, V.; Ramos, A.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724.
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalek, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317.
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.; Patiño, B. Mycotoxins|Toxicology, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 887–892.
- Pan, T.; Hsu, W. Monascus-Fermented products. In Encyclopedia of Food Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 815–825.
- Macholz, R. Some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Int. Agency Res. Cancer 1986, 32, 150.
- Sarı, F.; Öztaş, E.; Özden, S.; Özhan, G. Liquid chromatographic determination of citrinin residues in various meat products: A pioneer survey in Turkey. J. Fac. Pharm. Istanb. Univ. 2020, 50, 195–202.
- Silva, L.; Pereira, A.; Pena, A.; Lino, C. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2021, 10, 14.
- Wu, M.; Ayres, J.; Koehler, P. Production of citrinin by Penicillium viridicatum on country-cured ham. J. Appl. Microbiol. 1974, 27, 427–428.
- Meerpoel, C.; Vidal, A.; Tangni, E.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A study of carry-over and histopathological effects after chronic dietary intake of citrinin in pigs, broiler chickens and laying hens. Toxins 2020, 12, 719.
- Kharayat, B.; Singh, Y. Mycotoxins in foods: Mycotoxicoses, detection, and management. In Microbial Contamination Food Degradation; Academic Press: Cambridge, MA, USA, 2018; pp. 395–421.
- Bullerman, L. Mycotoxins|Classifications. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4080–4089.
- Bailly, J.; Tabuc, C.; Quérin, A.; Guerre, P. Production and stability of patulin, ochratoxin a, citrinin, and cyclopiazonic acid on dry cured ham. Rev. Anal. Chem. 2005, 68, 1516–1520.
- Viegas, C.; Nurme, J.; Piecková, E.; Viegas, S. Sterigmatocystin in foodstuffs and feed: Aspects to consider. Mycology 2018, 11, 91–104.
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B 2018, 1073, 191–200.
- Aupanun, S.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Poapolathep, A. An overview of the toxicology and toxicokinetics of fusarenon-X, a type B trichothecene mycotoxin. J. Vet. Med. Sci. 2017, 79, 6–13.
- Saito, M.; Horiuchi, T.; Ohtsubo, K.; Hatakana, Y.; Ueno, Y. Low tumor-incidence in rats with long-term feeding of fusarenon X, a cytotoxic trichothecene produced by Fusarium nivale. Jpn. J. Exp. Med. 1980, 50, 293–302.
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.; Kaushik, N.; Choi, E. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952.
- Nunez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sanchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120.
- Wang, Z.; Wu, Q.; Kuča, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: Signaling pathways and human exposure risk assessment—An update. Arch. Toxicol. 2014, 88, 1915–1928.
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148.
- Frisvald, J.C.; Samson, A. Mycotoxins produced by species of Penicillium and Aspergillus occurring in cereals. In Cereal Grain; Chelkwski, J., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 441–476.
- Pitt, J.I.; Leistner, L. Toxigenic Penicillium species. In Mycotoxins and Animal Foods; Smith, J.E., Henderson, R.S., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1991; pp. 81–100.
This entry is offline, you can click here to edit this entry!
