Acne vulgaris stands out as the most prevalent skin disorder among teenagers and young adults, causing physical discomfort and considerable economic and psychological burdens on individuals and society. Chemical peeling is a skin resurfacing technique designed to rebuild healthy skin using exfoliating substances, a simple and affordable process with various dermatological uses. Chemical peels, classified as superficial, medium, and deep, have been utilized for acne vulgaris and multiple other skin issues. In these chemical peels, a diverse range of chemical substances is employed, each with its unique mode of action. Among these, α-hydroxy and β-hydroxy acids have gathered attention for their efficacy in reducing acne lesions and enhancing overall skin appearance. Acids, such as salicylic acid, glycolic acid, or lactic acid, are commonly used in chemical peels due to their exfoliating and sebum-regulating properties. Despite the widespread use of these acids, there exists a lack of consensus regarding the most effective acid type and concentration for treating acne-prone skin.
1. Introduction
Acne vulgaris is the most common skin disease affecting adolescents and young adults. Although it is seen as a mild skin condition, it places a significant economic and psychological cost on individuals and society. Anxiety, depression, and poor self-esteem are common among patients suffering from acne vulgaris, negatively impacting their quality of life [
1,
2].
Propionibacterium acnes colonization, inflammation, enhanced keratinocyte proliferation in the follicular infundibulum, and hypersensitive androgen-sensitive sebaceous glands are some of the components involved in the pathogenesis of acne. It is a disease of the pilosebaceous units, clinically characterized by seborrhea, comedones, papules, pustules, nodules, cysts, and, in some cases, scarring [
3,
4].
Chemical peeling is a skin resurfacing technique frequently used for cosmetic and face rejuvenation. A new epidermal layer of the dermal tissues regenerates because the skin suffers controllable damage under the influence of a chemical agent. The amount of acid employed, the kind of vehicle, the amount of buffering, and the length of skin contact all affect how deep the peeling is. Chemical peels are divided into superficial (epidermis-papillary dermis), medium (papillary to upper reticular dermis), and deep peels (mid-reticular dermis), depending on how deeply they penetrate the skin [
14].
Medium-depth and superficial chemical peels are used frequently to treat acne vulgaris, as there are various substances and combinations of peels (alpha-hydroxy acids, beta-hydroxy acids, polyhydroxy acids, and bionic acids). Chemical peels can decrease sebum production and have antibacterial, anti-inflammatory, keratolytic, and comedolytic properties. Consequently, chemical peels have been utilized widely to treat acne vulgaris as an additional or maintenance therapy [
15,
16].
Although chemical peels are commonly used to treat acne, there is a lack of high-quality studies to support their efficacy and safety. Some small studies have shown promising results. Thus, there is a need for larger, randomized, controlled trials to understand better the effectiveness of chemical peels in treating acne. Additionally, there is a need for more research to determine the optimal type of chemical peel and concentration of the active ingredient for treating acne. The available studies have used a variety of chemical peels, including salicylic acid, glycolic acid, and trichloroacetic acid, and the optimal concentration and duration of treatment may vary depending on the individual and the severity of the acne. Furthermore, there is a need for more research to determine the safety of chemical peels in treating acne, particularly in individuals with darker skin tones who may be at a higher risk of hyperpigmentation or scarring [
17,
18].
3. Organic Acids Used in Chemical Peels for Acne Treatment
3.1. Classification of the Chemical Peel Agents Based on the Depth of Penetration
Chemical peeling agents can be classified based on the depth of penetration into the skin; there are three main types of chemical peels based on the depth of penetration: superficial, medium, and deep. The characteristics of these types of peeling are presented below.
3.1.1. Superficial Peels
Superficial peels penetrate only the outermost layer of the skin (epidermis) and are used to improve skin texture, reduce fine lines and wrinkles, and treat mild acne. These peels involve the application of a chemical solution to the skin’s surface, which helps to exfoliate dead skin cells, unclog pores, and stimulate the growth of new, healthier skin. These peels use weak acids like α-hydroxy acids (AHAs), such as glycolic acid and lactic acid, and β-hydroxy acids (BHAs), such as salicylic acid, to exfoliate the skin. Superficial peels have minimal downtime and are generally safe for all skin types [
19,
20].
3.1.2. Medium-Depth Peels
Medium-depth peels penetrate the epidermis and upper layers of the dermis and treat more severe skin concerns such as sun damage, fine lines and wrinkles, and acne scars. Medium chemical peels are a more aggressive form of chemical peel compared to superficial peels. They are sometimes used to manage acne vulgaris, particularly for individuals with more severe or stubborn acne and acne scarring. These peels use trichloroacetic acid (TCA), Jessner’s solution, or a combination of TCA and glycolic acid to exfoliate the skin. Medium-depth peels have a more extended downtime and may require pain relief treatment during the procedure [
21,
22].
3.1.3. Deep Peels
Deep peels penetrate the deeper layers of the dermis and are used to treat severe skin concerns such as deep wrinkles, scarring, and severe sun damage. These peels use phenol, TCA, or a combination of TCA and phenol to exfoliate the skin. Deep peels have the most extended downtime and are generally only recommended for fair-skinned individuals due to the risk of hypopigmentation or other adverse effects. Deep peels can also determine the appearance of second-degree burns; applying the solution is preferred based on phenols in this condition. The treatment must be used only once and with the utmost caution due to adverse reactions (the appearance of spots that will not disappear); this method is recommended only for people with severe dermatological problems and should only be performed by a dermatologist [
22,
23].
3.1.4. Peeling Agents
Among the factors that influence the penetration capacity of the tissues by the chemical substances are the pH of the peeling agent, the number of layers applied, and the time given for the action before the substance is neutralized. The penetration depth is determined by the peeling agent’s nature, concentration, and pH.
4. Classification of the Chemical Peel Agents Based on Chemical Structure
4.1. Aliphatic Carboxylic Acids
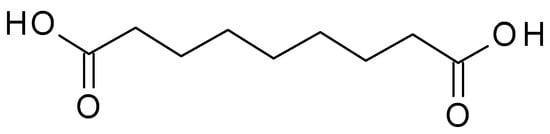
4.1.1. Azelaic Acid
Azelaic acid (C
9H
16O
4) (
Figure 1) is a naturally occurring dicarboxylic acid found in whole grain cereals, wheat, rye, and barley. It is a straight-chain, saturated fatty acid with a carbon chain length of nine carbon atoms. It is a white crystalline powder with a slight odour, sparingly soluble in water but more soluble in organic solvents. The pKa value of azelaic acid is around 4.55, indicating that it exists mainly in its non-ionized form at physiological pH. The pH of azelaic acid formulations typically ranges from 4 to 5, which is close to the skin’s natural pH level [
25].
Figure 1. Azelaic acid chemical structure and the IUPAC name: nonanedioic acid.
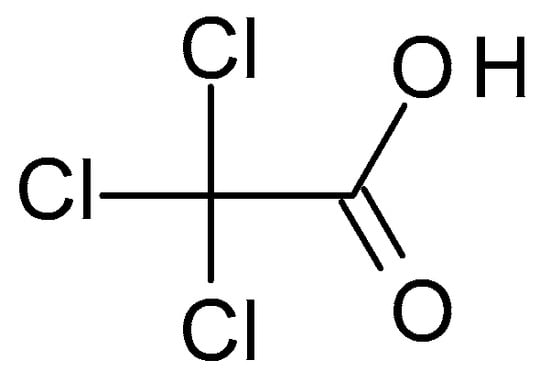
4.1.2. Thricloracetic Acid (TCA)
TCA (C
2HCl
3O
2) (
Figure 2) is a structural analogue of acetic acid in which the hydrogen atoms in the methyl group have been replaced with chlorine atoms. It is a white crystalline solid highly soluble in water and alcohol. TCA has a pH of around 1.5 to 2, which makes it highly acidic [
28].
Figure 2. Thricloracetic acid chemical structure and the IUPAC name: 2,2,2-trichloroacetic acid.
4.2. Aliphatic Hydroxycarboxylic Acids
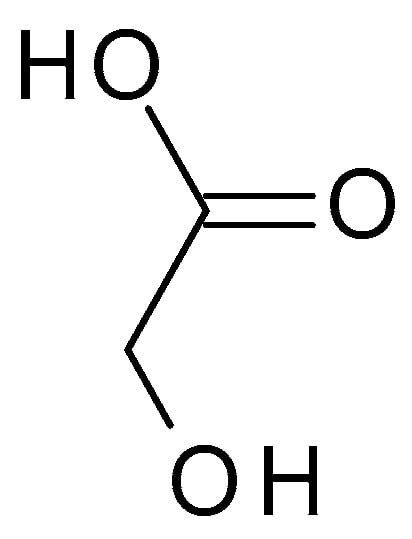
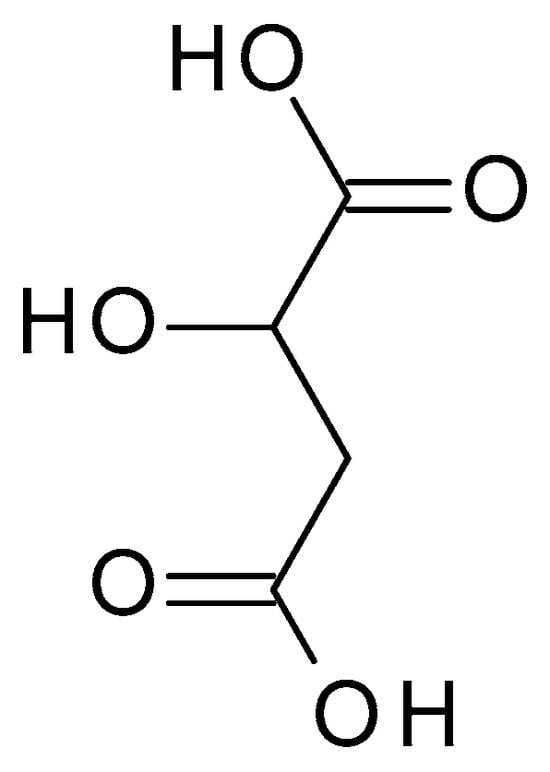
4.2.1. Glycolic Acid
Glycolic acid (C
2H
4O
3) (
Figure 3) is the simplest AHA with the smallest molecular weight and size. It is a colourless, odourless, crystalline substance highly soluble in water. This AHA can be isolated from natural sources, such as sugarcane, sugar beets, pineapple, or cantaloupe. Glycolic acid has a pH of around 3.5 to 4, which makes it mildly acidic [
32].
Figure 3. Glycolic acid chemical structure and the IUPAC name: 2-hydroxyacetic acid.
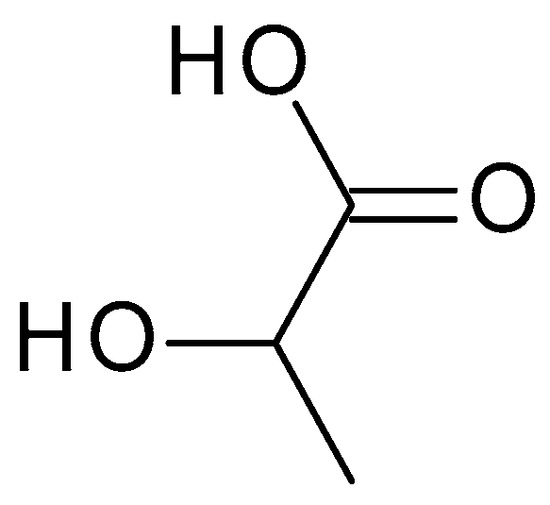
4.2.2. Lactic Acid
Lactic acid (C
3H
6O
3) (
Figure 4) is structurally similar to glycolic acid, having an additional methyl group. Lactic acid is produced via the bacterial fermentation of milk or carbohydrates. It is a colourless or yellowish liquid with a slightly acidic odour. The pH of lactic acid is around 3.5 to 4, which makes it mildly acidic [
36].
Figure 4. Lactic acid chemical structure and the IUPAC name: 2-hydroxypropanoic acid.
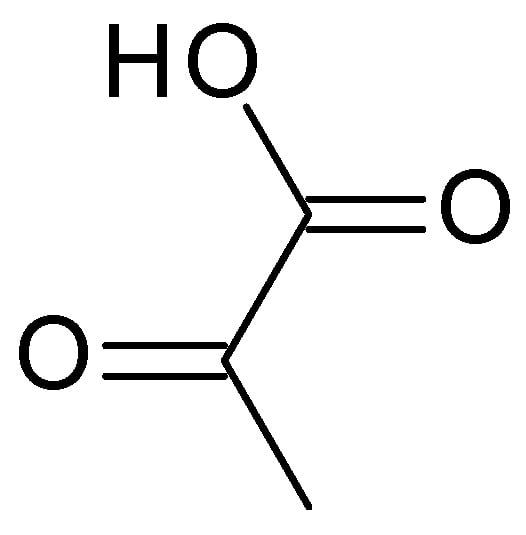
4.2.3. Pyruvic Acid
Pyruvic acid (C
3H
4O
3) (
Figure 5) is the simplest α-keto acid, with a functional ketone and carboxylic acid group. Pyruvic acid is a colourless to pale yellow liquid with a distinct odour, highly soluble in water and alcohol. Typically, it has a pH range of 2–3 when dissolved in water. It plays a crucial role in various biological processes, including energy metabolism and the synthesis of amino acids [
39].
Figure 5. Pyruvic acid chemical structure.
4.2.4. Ascorbic Acid
Ascorbic acid (C
6H
8O
6) (Vitamin C) (
Figure 6) is a water-soluble vitamin that plays a crucial role in various bodily functions. Ascorbic acid is susceptible to degradation, particularly under certain conditions such as exposure to heat, light, and oxygen. It is relatively unstable and can undergo oxidation, leading to the formation of dehydroascorbic acid. Ascorbic acid is an organic acid with a relatively low pKa (4.2); it can act as a weak acid, donating hydrogen ions in solution and lowering the pH. It is a good reducing agent and undergoes reversible oxidation-reduction reactions; it can donate electrons, making it an effective antioxidant that can scavenge and neutralize free radicals. Free radicals can contribute to inflammation and damage the skin’s barrier, worsening acne symptoms. It is an essential nutrient; the human body cannot synthesize it, and it must be obtained via diet or supplements. Ascorbic acid helps protect the skin against free radical damage caused by environmental factors such as UV radiation, pollution, and oxidative stress; this antioxidant activity can help prevent premature ageing and support overall skin health [
41].
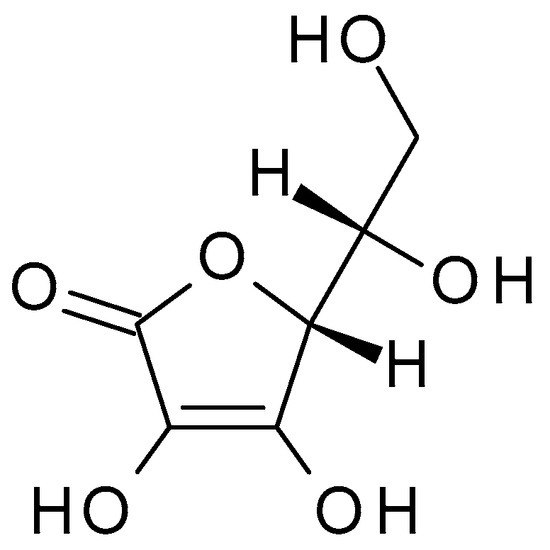
Figure 6. Ascorbic acid chemical structure and the IUPAC name: ((2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one).
4.2.5. Citric Acid
Citric acid (C
6H
8O
7) (2-hydroxypropane-1,2,3-tricarboxylic acid) (
Figure 7) is a weak organic acid with three carboxylic acid functional groups naturally found in citrus fruits. Citric acid is a white crystalline powder with a tart, acidic taste, which contributes to the sourness of citrus fruits; it is highly soluble in water. Citric acid has a pH value of approximately 2.2 to 2.5 in a 1% aqueous solution. Citric acid has antioxidant properties, donating electrons to neutralize free radicals and prevent their harmful effects [
43].
Figure 7. Citric acid chemical structure and the IUPAC name: 2-hydroxypropane-1,2,3-tricarboxylic acid.
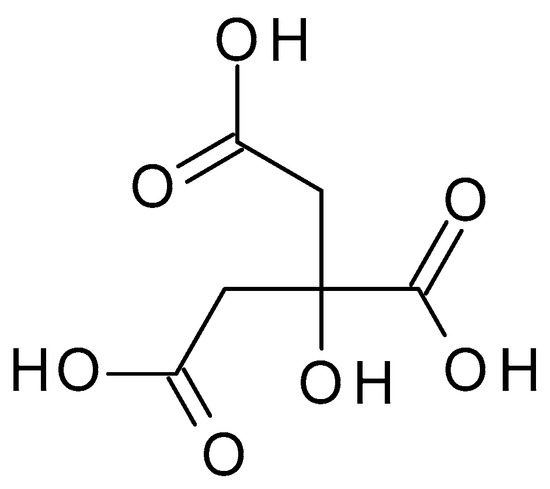
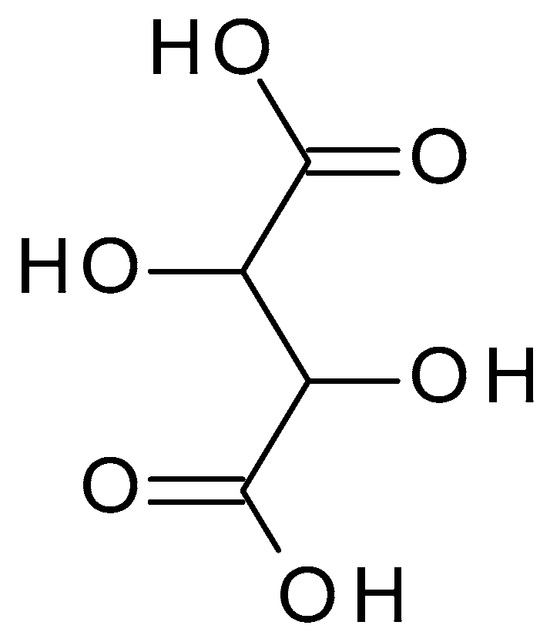
4.2.6. Malic Acid
Malic acid (C
4H
6O
5) (
Figure 8) is a dicarboxylic acid occurring naturally in various fruits, such as apples, grapes, and citrus. Malic acid is a white crystalline powder with a pleasantly sour taste and is highly soluble in water. Malic acid has a pH value of approximately 2.83 in aqueous solution [
45].
Figure 8. Malic acid chemical structure and the IUPAC name: 2-hydroxybutanedioic acid.
4.2.7. Tartaric Acid
Tartaric acid (C
4H
6O
6) (2,3-dihydroxybutanedioic acid) (
Figure 9) is a dicarboxylic acid occurring naturally in various fruits, such as grapes or bananas. Tartaric acid is a white crystalline powder with a sour taste; it is highly soluble in water. It has a pH value of approximately 2.2 to 3.0 in aqueous solution [
47].
Figure 9. Tartaric acid chemical structure and the IUPAC name: 2,3-dihydroxybutanedioic acid.
4.3. Aromatic Hydroxycarboxylic Acids
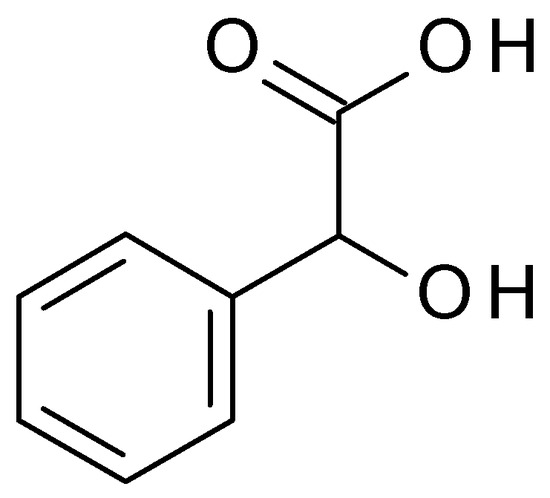
4.3.1. Mandelic Acid
Mandelic acid (C
8H
8O
3) (2-hydroxy-2-phenylacetic acid) (
Figure 10) is an AHA derived from bitter almonds. Mandelic acid is a white crystalline powder, highly soluble in water. Mandelic acid has a pH value of approximately 2.8 to 3.2 in aqueous solution [
48].
Figure 10. Mandelic acid chemical structure and the IUPAC name: 2-hydroxy-2-phenylacetic acid.
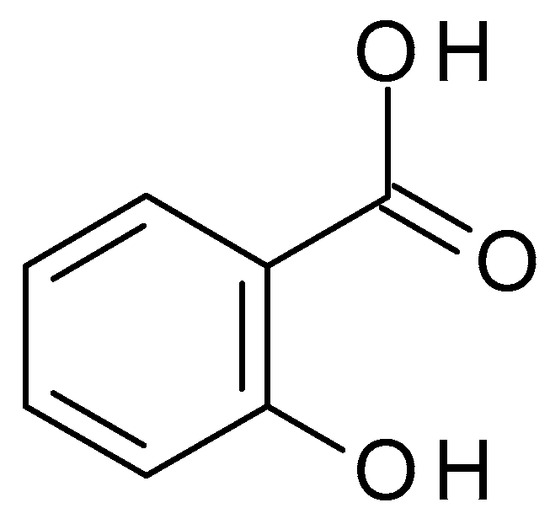
4.3.2. Salicylic Acid
Salicylic acid (C
7H
6O
3) (
Figure 11) is a β-hydroxy acid (BHA) obtained from the bark of the white willow and wintergreen leaves. Salicylic acid is a white crystalline powder, slightly soluble in water but highly soluble in organic solvents. Salicylic acid is a weak acid with a pH value of approximately 2.4 in aqueous solution. It possesses keratolytic, fungicidal, and bacteriostatic effects. Salicylates are acetylsalicylic acid salts and are employed as analgesics [
50].
Figure 11. Salicylic acid chemical structure and the IUPAC name: 2-hydroxybenzoic acid.
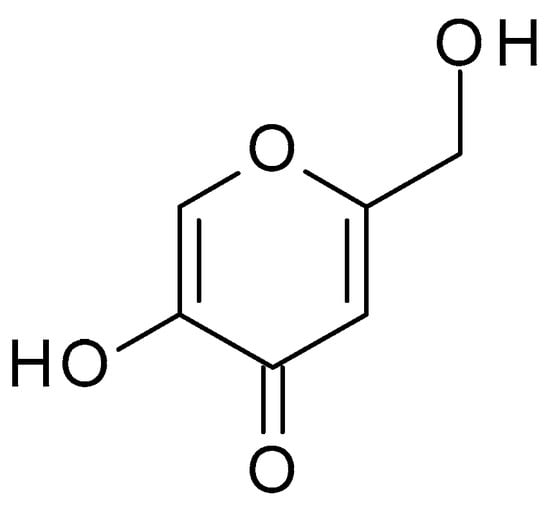
4.3.3. Kojic Acid
Kojic acid (C
6H
6O
4) (
Figure 12) is a naturally occurring compound derived from several species of fungi, especially
Aspergillus oryzae. In the fermentation of rice used to make sake (a Japanese alcoholic rice drink), kojic acid is obtained as a byproduct. Kojic acid is a white crystalline powder, sparingly water-soluble, well soluble in organic solvents. It is more stable in acidic conditions (pH 3–5) and less stable in alkaline conditions; it can undergo degradation when exposed to heat, light, and air. Kojic acid has chelating properties, which means it can bind to metal ions such as copper and iron. This property contributes to its ability to inhibit the activity of tyrosinase, an enzyme involved in melanin production. It is commonly used in the cosmetic and skincare industry for its skin-lightening and brightening properties [
54,
55].
Figure 12. Kojic acid chemical structure and the IUPAC: 5-hydroxy-2-(hydroxymethyl)pyran-4-one.
4.4. Polyhydroxy Acids (PHAs)
PHAs are a group of chemical compounds used in skincare peels. PHA peels offer a milder alternative to traditional chemical peels, providing exfoliation and various skin benefits [
57].
PHA peels provide gentle skin exfoliation, helping remove dead skin cells, unclog pores, and promote cell turnover. PHAs have larger molecular sizes than AHAs and BHAs, making them less likely to penetrate deeply into the skin. As a result, PHA peels are generally considered gentler and more suitable for sensitive skin types [
58]. PHAs are frequently combined with additional compounds, including antioxidants, soothing agents, or hydrating components, to maximize the overall effects of the peel and reduce any potential irritation or dryness [
57].
Generally, PHAs are well-tolerated by different skin types, including sensitive skin, as they have a lower potential for causing irritation or stinging sensations than other peeling agents. PHAs have humectant properties, meaning they can attract and retain moisture in the skin. Consequently, the PHAs can help improve skin hydration and reduce dryness or flakiness commonly experienced after chemical peels. Often, PHA peels are recommended for individuals with dry or dehydrated skin [
58].
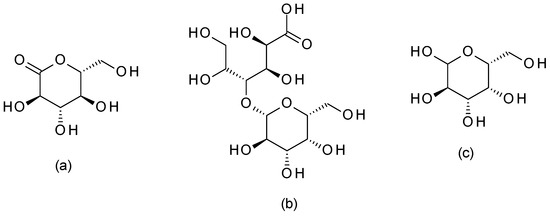
The most used PHAs are galactose, gluconolactone, and lactobionic acid, briefly presented below.
4.4.1. Galactose
Galactose ((3
R,4
S,5
R,6
R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol) (
Figure 13) is a PHA sugar molecule. It offers mild exfoliation and can help improve skin hydration and texture. Galactose has humectant properties, helping attract and retain skin moisture [
59].
Figure 13. Polyhydroxy acids chemical structures ((
a)—gluconolactone; (
b)—lactobionic acid; (
c)—galactose).
4.4.2. Gluconolactone
Gluconolactone ((3
R,4
S,5
S,6
R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-one) (
Figure 13) is a PHA derived from glucose. This PHA has a larger molecular size than other hydroxy acids, making it gentle on the skin and offering exfoliation, hydration, and antioxidant properties. It can help improve the skin’s barrier function [
59].
4.4.3. Lactobionic Acid
Lactobionic acid ((2
R,3
R,4
R)-2,3,5,6-tetrahydroxy-4-[[(2
S,3
R,4
S,5
R,6
R)-3,4,5-trihydroxy-6-(hydroxymethyl)-2-tetrahydropyranyl]oxy]hexanoic acid) (
Figure 13) is another PHA commonly used in peels derived from lactose, and it offers similar benefits to gluconolactone. Lactobionic acid provides gentle exfoliation, moisturization, and antioxidant effects and can help improve skin texture and tone [
60].
5. Combined Peeling Formulas
Combined peeling formulas often utilize a combination of different acids to provide comprehensive exfoliation and address multiple skin concerns. The specific combinations of acids can vary depending on the desired outcomes and the individual’s skin type and needs. Some peeling formulas combine multiple acids in specific ratios to provide a balanced and customized approach. For example, a combination peel may include a blend of AHAs, BHAs, and PHAs to simultaneously address various skin concerns, such as acne, hyperpigmentation, and texture [
61]. Usually, a mixture of AHAs and beta-hydroxy acids BHAs, such as glycolic acid, lactic acid, and salicylic acid, can target multiple aspects of acne. AHAs exfoliate the skin’s surface, while BHAs penetrate the pores to unclog them. This combination can help with acne-related concerns like uneven skin tone [
62].
Salicylic acid and glycolic acid peel effectively treat active acne and exfoliate the skin. Salicylic acid penetrates the pores to remove excess oil and unclog them. In contrast, glycolic acid exfoliates the outer layer of the skin, promoting cell turnover and reducing the appearance of acne scars. This combination can improve skin texture and reduce acne breakouts [
63]. Salicylic acid combined with mandelic acid peel combines the pore-clearing and anti-inflammatory properties of salicylic acid with the gentle exfoliation and antibacterial effects of mandelic acid, helping to unclog pores, reduce acne-causing bacteria, and improve overall skin texture [
64].
Salicylic acid and trichloroacetic (TCA) peel can be used for more severe acne and scarring. TCA is a stronger peeling agent that can penetrate deeper layers of the skin, stimulating collagen production and improving the appearance of deeper scars, while salicylic acid complements TCA by targeting active acne and surface imperfections [
65]
Also, salicylic acid combined with retinol peeling formula incorporates the pore-clearing benefits of salicylic acid with the skin-renewing effects of retinol. It helps unclog pores, regulate oil production, and promote cellular turnover for smoother skin [
66].
Glycolic acid combined with lactic acid and azelaic acid peeling formula incorporates multiple AHAs to exfoliate the skin, reduce acne scars and hyperpigmentation, and promote cell turnover. Azelaic acid further contributes to its antibacterial and anti-inflammatory properties, making it efficient for acne-prone skin [
67].
The most popular combined peeling formula is Jessner’s peel, named after the American dermatologist Dr. Max Jessner, who developed the formula in the early 20th century. This peeling formula can address various skin concerns, including acne, acne scars, sun damage, fine lines, and uneven skin tone. It is a medium-depth peel combining three key ingredients: resorcinol, lactic acid, and salicylic acid. The specific concentrations of these ingredients in a Jessner’s peel can vary depending on the patient’s skin type and the desired results. Usually, the composition of the formula is customized to meet individual needs, adjusting the strength of each component as necessary [
69].
Jessner’s peel exfoliates the outer layer of the skin, helping to remove dead skin cells, unclog pores, and clear away debris, preventing the formation of comedones and reducing the occurrence of acne breakouts. Salicylic acid, one of the main components of Jessner’s peel, can penetrate the pores and dissolve excess oil. It can help prevent the accumulation of sebum. The combination of lactic acid and salicylic acid provides anti-inflammatory properties. In addition, this combination can help to calm existing acne lesions, reduce redness, and alleviate inflammation. Jessner’s peel promotes the regeneration of new skin cells and stimulates collagen production, resulting in improved skin texture, smoother appearance, and a more even skin tone. Also, it can help reduce the appearance of acne scars and hyperpigmentation caused by acne. Jessner’s peel is a controlled treatment that can be adjusted based on the individual’s skin type and condition. The applied number of layers and the duration of the peel can be customized to address specific acne concerns and minimize the risk of adverse effects [
70,
71,
72].
6. Comparative Studies between the Effectiveness and Safety of Different Acids in Chemical Peels on Acne Skin
6.1. Comparative Studies that Used Azelaic acid Versus Other Peeling Agents
Abdel Hay et al. (2019) compared the efficacy of a combined solution of 20% azelaic acid and 20% salicylic acid versus a 25% TCA peel for treating acne. The study included 34 participants who received four treatment sessions two weeks apart. One side of each patient’s face was treated with the combined solution of salicylic acid and azelaic acid, while the other side was treated with a 25% TCA peel. The results were assessed based on physician-reported clinical improvement, a dermoscopic assessment of erythema, and patient satisfaction. By the end of the study, both treatments led to a significant improvement in acne, with no significant difference in clinical improvement. Patients reported more discomfort on the TCA-treated side. The combination of salicylic acid and azelaic acid is recommended for early-stage treatment if patients have more inflammatory lesions. In comparison, TCA is recommended if patients have more non-inflammatory lesions. Patients reported greater satisfaction with the salicylic acid–azelaic acid-treated side, suggesting that this combination may offer a more comfortable and satisfactory treatment experience than TCA [
75].
6.2. Comparative Studies that Used Glycolic Acid Versus Other Peeling Agents
Kim S.W. et al. (1999) compared the efficiency and safety of glycolic acid and Jessner peels on mild to moderately severe facial acne. Subsequently, 26 individuals were treated simultaneously with 70% glycolic acid and Jessner’s solution twice a week on each side of the face. A blinded evaluator, who did not know the randomization code, conducted evaluations of the therapy on the randomized treatment sides. All participants were questioned about how their facial acne had improved and if they encountered adverse effects; they also responded to the preference test for the two peeling techniques. Most patients’ acne severity did not alter after the first therapy session. However, 50% of the patients who received either glycolic acid or Jessner’s solution peels after the third session exhibited improvement. Both peels showed improvement in acne after multiple sessions, but Jessner’s solution had more side effects like erythema and exfoliation [
77].
El Refaei et al. (2015) evaluated the efficacy and tolerability of a combination of 20% salicylic–10% mandelic acid peel compared to a 35% glycolic acid peel in the treatment of active acne vulgaris, post-acne scarring, and associated hyperpigmentation. The study included 40 participants with facial acne vulgaris who were randomly divided into two groups and underwent seven peeling sessions every two weeks. Both peels effectively treated inflammatory acne, noninflammatory acne, post-acne hyperpigmentation, and certain acne scars. Salicylic acid–mandelic acid peel showed higher efficacy in several acne parameters than glycolic acid peel [
80].
Also, in a split face study, Manjhi M. et al. (2020) studied the comparative efficacy of 50% glycolic acid peel and 30% salicylic acid peel. Thirty participants were involved in the study and underwent six sessions every two weeks, with one side of the face treated with glycolic acid and the other with salicylic acid. Both peels were effective, but salicylic acid peel was more effective and responded faster in treating mild to moderate acne vulgaris. This enhanced effectiveness of the salicylic acid peel may be attributed to its lipophilic nature, which allows it to penetrate the sebaceous glands more effectively [
82].
6.3. Comparative Studies that Used Salicylic Acid Versus Other Peeling Agents
Bae B.G. et al. (2013) compared the efficacy and safety of 30% salicylic acid peels with Jessner’s solution peels in treating mild-to-moderate facial acne. A total of 13 male participants with facial acne were enrolled in the study. One side of each patient’s face was treated with Jessner’s solution, while the other was treated with 30% salicylic acid. Three sessions of peels were administered at 2-week intervals. A blinded evaluator counted noninflammatory and inflammatory lesions before and after each treatment. Inflammatory acne lesion counts did not differ significantly between salicylic acid and Jessner’s solution peels. The study concludes that salicylic acid peels were effective for treating inflammatory acne lesions and were more effective than Jessner’s solution peels for treating noninflammatory acne lesions [
84].
Dayal S. et al. (2017) compared the efficacy and safety of 30% salicylic acid with Jessner solution in mild-to-moderate acne vulgaris. A total of 40 participants with mild-to-moderate acne were enrolled in the study and were randomly divided into two groups, one treated with 30% salicylic acid peels and the other with Jessner solution peels, which were conducted two weeks apart for six peels. The study concludes that salicylic peels were more effective than Jessner peels in treating noninflammatory lesions, specifically comedones, and improving mild-to-moderate facial acne vulgaris [
85].
6.4. Comparative Studies that Used Pyruvic Acid Versus Other Peeling Agents
Zdrada J. et al. (2020) compared the effect of a combined peeling formula containing pyruvic acid with a mixture of glycolic and salicylic acids. Fourteen women with acne were involved in the study. Four treatments were applied at two-week intervals. A preparation containing 50% pyruvic acid was used on one side of the face, while on the other, a mixture of glycolic and salicylic was applied. The skin moisture, sebum secretion, and skin tone were evaluated. It was concluded that pyruvic acid increased skin hydration and reduced skin colour. Regarding acne treatment, therapeutic effects were comparable between the acids and high-concentration pyruvic acid mixture. Using a combination of acids in chemical peels yielded fewer side effects than using a single acid in a high concentration [
88].
6.5. Comparative Studies that Used Mandelic Acid Versus Other Peeling Agents
Jartarkar et al. (2017) compared the effectiveness of 20% salicylic acid and 30% mandelic acid peels in treating mild to moderately severe acne vulgaris. Fifty participants with mild to moderately severe acne were randomly divided into two groups. The participants underwent six sessions of peeling treatment at two-week intervals. Salicylic acid peel was more effective in reducing inflammatory and noninflammatory acne lesions than mandelic acid peel. However, mandelic acid peel had fewer side effects and did not lead to postinflammatory hyperpigmentation. These results suggest that salicylic acid may offer slightly better results in improving acne lesions, but mandelic acid could be preferable for individuals concerned about side effects like hyperpigmentation [
64].
7. Complications of Chemical Peels with Acids
While acid chemical peels can effectively treat acne, there are potential complications and side effects, mainly if the peels are not administered correctly or if the skin is not adequately prepared or cared for before and after the treatment. The severity and frequency of side effects can vary depending on factors such as the type and concentration of acids used, individual skin sensitivity, and the skill of the practitioner administering the peel [
14,
17].
Table 3 shows some common side effects associated with chemical peels for acne.
Table 3. Adverse effects observed after chemical peels with acids on acne skin [
14,
17,
90,
91].
| Adverse Effects |
Management |
Comments |
| Skin irritation and redness |
- -
-
These symptoms typically subside within a few days to a week;
- -
-
Application of soothing, hydrating moisturizer to the treated area to alleviate redness and reduce irritation;
- -
-
Avoid using harsh skincare products or exfoliants until the skin has fully healed.
|
- -
-
Following a chemical peel, it is expected to experience temporary skin irritation, redness, and increased sensitivity;
- -
-
Individuals with sensitive skin or those who undergo deeper peels may experience more pronounced redness and prolonged irritation.
|
| Post-inflammatory hyperpigmentation |
- -
-
Proper sun protection and skin-lightening agents may help minimize the risk of post-inflammatory hyperpigmentation.
|
- -
-
Chemical peels can sometimes trigger post-inflammatory hyperpigmentation, especially in individuals with darker skin tones.
|
| Peeling and flaking |
- -
-
Avoid picking or forcefully removing the peeling skin to prevent potential complications;
- -
-
Application of a gentle, non-abrasive moisturizer to keep the skin hydrated and minimize dryness;
- -
-
Avoid harsh cleansers or exfoliants that may further irritate the peeling skin.
|
- -
-
After chemical peels, the treated skin may peel or flak as the outermost layers slough off, resulting in temporary dryness and skin shedding.
|
| Increased sensitivity to sunlight |
- -
-
It is crucial to protect the treated area from excessive sun exposure and use a broad-spectrum sunscreen with a high SPF to minimize the risk of sunburn and hyperpigmentation;
- -
-
Sun protection should be continued for several weeks following the peel;
- -
-
Wearing protective clothing to shield the treated area from the sun further;
|
- -
-
Chemical peels can make the skin more sensitive to sunlight.
|
| Allergic reactions |
- -
-
It is essential to undergo a patch test before the complete treatment to identify potential allergies.
|
- -
-
Individuals may be allergic or sensitive to certain acids used in chemical peels—can manifest as redness, itching, swelling, or even more severe symptoms.
|
| Infection |
- -
-
Follow the instructions provided by the dermatologist or skincare professional and keep the treated area clean and protected
|
- -
-
Proper hygiene is not maintained during the treatment, or if the skin is not adequately protected post-peel, there is a risk of infection
|
| Post-treatment complications |
- -
-
Follow any specific instructions or treatment recommendations provided by the dermatologist to address these complications
|
- -
-
In rare cases, chemical peels can lead to scarring, changes in skin colour, or infection—complications are more commonly associated with deep peels or when the peels are administered incorrectly or by untrained individuals.
|
This entry is adapted from the peer-reviewed paper 10.3390/molecules28207219