The composition of the gut microbiota represents an early indicator of chronic post-radiation side-effects in elderly bone and immunogenic traits of the gastrointestinal homeostasis. Fecal microbiota analyses revealed that the relative abundances of Bacteroides massiliensis, Muribaculum sp., or Prevotella denticola were different between conventional microbiota (CM) and anti-inflammatory restricted microbiota (RM). The murine RM was found conditional on mucosa-associated dysbiosis under both, disturbances of interleukin (IL)-17 signaling, and exposure to radiation alone. The hypothesis that intestinal microbiota induced alterations in DNA repair and expressed transforming growth factor (TGF)-β in the small intestine is discussed, thereby impacting bone microstructure and osteoblast dysfunction in silicon ion (1.5 Gy 28Si ions of 850 MeV/u) irradiated mice. Bacterial microbiota compositions influenced therapeutic approaches, correlated with clinical outcomes in radiotherapy and were associated with alterations of the immune response to severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infections during the last global pandemics.
- TGF-beta
- radiation
- small intestine
- enteropathy
- antitumor immunity
1. Introduction
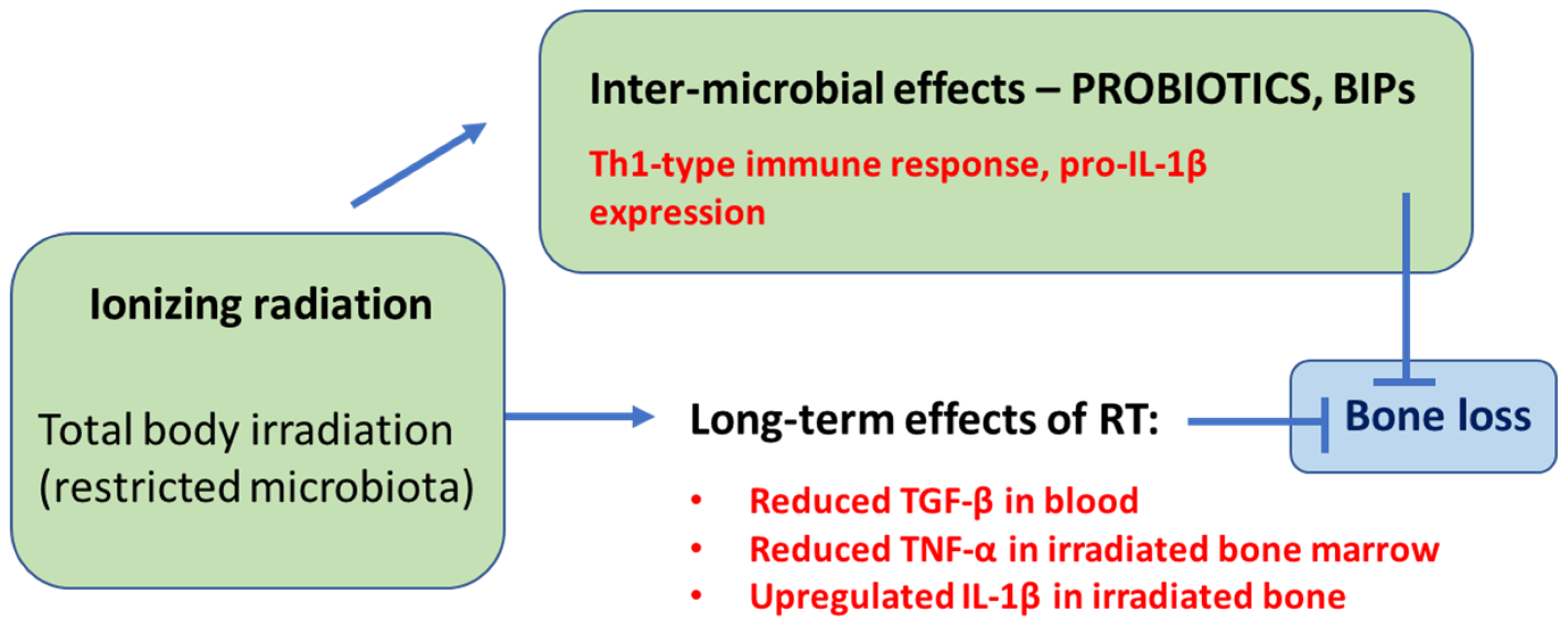
2. Different Gut Microbiota Can Both Negatively and Positively Impact Radiation-Induced Bone Loss
3. SARS-CoV-2 Infections Impact Radio-Immunogenic Responses of the Gastrointestinal Tract
This entry is adapted from the peer-reviewed paper 10.3390/microbiolres14020048
References
- Maier, I.; Berry, D.M.; Schiestl, R.H. Intestinal microbiota reduces genotoxic endpoints induced by high-energy protons. Radiat. Res. 2014, 181, 45–53.
- Maier, I.; Liu, J.; Ruegger, P.M.; Deutschmann, J.; Patsch, J.M.; Helbich, T.H.; Borneman, J.; Schiestl, R.H. Intestinal bacterial indicator phylotypes associate with impaired DNA double-stranded break sensors but augmented skeletal bone micro-structure. Carcinogenesis 2020, 41, 483–489.
- Maier, I.; Ruegger, P.M.; Deutschmann, J.; Helbich, T.H.; Pietschmann, P.; Schiestl, R.H.; Borneman, J. Particle Radiation Side-Effects: Intestinal Microbiota Composition Shapes Interferon-γ-Induced Osteo-Immunogenicity. Radiat. Res. 2022, 197, 184–192.
- Cheema, A.K.; Maier, I.; Dowdy, T.; Wang, Y.; Singh, R.; Ruegger, P.M.; Borneman, J.; Fornace, A.J., Jr.; Schiestl, R.H. Chemopreventive Metabolites Are Correlated with a Change in Intestinal Microbiota Measured in A-T Mice and Decreased Carcinogenesis. PLoS ONE 2016, 11, e0151190.
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661.
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, K.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682.
- Sjogren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367.
- Yu, M.; Tyagi, A.M.; Li, J.-Y.; Adams, J.; Denning, T.L.; Weitzmann, N.M.; Jones, R.M.; Pacifici, R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat. Commun. 2020, 11, 468.
- Masoud, G.N.; Wang, J.; Chen, J.; Miller, D.; Li, W. Design, Synthesis and Biological Evaluation of Novel HIF1α Inhibitors. Anticancer Res. 2015, 35, 3849–3859.
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257.
- Huang, T.; Wei, B.; Velazquez, P.; Borneman, J.; Braun, J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naïve CD4+ T lymphocytes. Clin. Immunol. 2005, 117, 221–230.
- Yamamoto, M.L.; Maier, I.; Dang, A.T.; Berry, D.; Liu, J.; Ruegger, P.M.; Yang, J.I.; Soto, P.A.; Presley, L.L.; Reliene, R.; et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013, 73, 4222–4232.
- Fujiwara, D.; Wei, B.; Presley, L.L.; Brewer, S.; McPherson, M.; Lewinski, M.A.; Borneman, J.; Braun, J. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J. Immunol. 2008, 180, 5843–5852.
- Wei, B.; Wingender, G.; Fujiwara, D.; Chen, D.Y.H.; McPherson, M.; Brewer, S.; Borneman, J.; Kronenberg, M.; Braun, J. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 2010, 184, 1218–1226.
- Vetizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084.
- Presley, L.L.; Wei, B.; Braun, J.; Borneman, J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010, 76, 936–941.
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901.
- Allais, L.; Kerckhof, F.M.; Verschuere, S.; Bracke, K.R.; De Smet, R.; Laukens, D.; Van den Abbeele, P.; De Vos, M.; Boon, N.; Brusselle, G.G.; et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 2016, 18, 1352–1363.
- Alwood, J.S.; Shahnazari, M.; Chicana, B.; Schreurs, A.S.; Kumar, A.; Bartolini, A.; Shirazi-Fard, Y.; Globus, R.K. Ionizing Radiation Stimulates Expression of Pro-Osteoclastogenic Genes in Marrow and Skeletal Tissue. J. Interferon Cytokine Res. 2015, 35, 480–487.
- Shen, V.; Birchman, R.; Xu, R.; Lindsay, R.; Dempster, D.W. Short-term changes in histomorphometric and biochemical turnover markers and bone mineral density in estrogen-and/or dietary calcium-deficient rats. Bone 1995, 16, 149–156.
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107.
- Hemdan, N.Y. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol. Lett. 2013, 149, 123–133.
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell Biol. 2000, 20, 8783–8792.
- Ruifrok, A.C.; Mason, K.A.; Lozano, G.; Thames, H.D. Spatial and temporal patterns of expression of epidermal growth factor, transforming growth factor alpha and transforming growth factor beta 1-3 and their receptors in mouse jejunum after radiation treatment. Radiat. Res. 1997, 147, 1–12.
- Booth, D.; Haley, J.D.; Bruskin, A.M.; Potten, C.S. Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int. J. Cancer 2000, 86, 53–59.
- Potten, C.S.; Booth, D.; Haley, J.D. Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br. J. Cancer 1997, 75, 1454–1459.
- Polistena, A.; Johnson, L.B.; Röme, A.; Wittgren, L.; Bäck, S.; Osman, N.; Molin, G.; Adawi, D.; Jeppsson, B. Matrilysin expression related to radiation and microflora changes in murine bowel. J. Surg. Res. 2011, 167, e137–e143.
- Gu, S.; Zaidi, S.; Hassan, M.I.; Mohammad, T.; Malta, T.M.; Noushmehr, H.; Nguyen, B.; Crandall, K.A.; Srivastav, J.; Obias, V.; et al. Mutated CEACAMs Disrupt Transforming Growth Factor Beta Signaling and Alter the Intestinal Microbiome to Promote Colorectal Carcinogenesis. Gastroenterology 2020, 158, 238–252.
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341.
- Gueulette, J.; Slabbert, J.P.; Böhm, L.; De Coster, B.M.; Rosier, J.F.; Octave-Prignot, M.; Ruifrok, A.; Schreuder, A.N.; Wambersie, A.; Scalliet, P.; et al. Proton RBE for early intestinal tolerance in mice after fractionated irradiation. Radiother. Oncol. 2001, 61, 177–184.
- Gueulette, J.; Bohm, L.; Slabbert, J.P.; De Coster, B.M.; Rutherfoord, G.S.; Ruifrok, A.; Octave-Prignot, M.; Binns, P.J.; Schreuder, A.N.; Symons, J.E.; et al. Proton relative biological effectiveness (RBE) for survival in mice after thoracic irradiation with fractionated doses. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1051–1058.
- Rodríguez-Tomàs, E.; Acosta, J.C.; Torres-Royo, L.; De Febrer, G.; Baiges-Gaya, G.; Castañé, H.; Jiménez, A.; Vasco, C.; Araguas, P.; Gómez, J.; et al. Effect of Low-Dose Radiotherapy on the Circulating Levels of Paraoxonase-1-Related Variables and Markers of Inflammation in Patients with COVID-19 Pneumonia. Antioxidants 2022, 11, 1184.
- Algara, M.; Arenas, M.; Marin, J.; Vallverdu, I.; Fernandez-Letón, P.; Villar, J.; Fabrer, G.; Rubio, C.; Montero, A. Low dose anti-inflammatory radiotherapy for the treatment of pneumonia by covid-19: A proposal for a multi-centric prospective trial. Clin. Transl. Radiat. Oncol. 2020, 24, 29–33.
- Montero, M.; Arenas, M.; Algara, M. Low-dose radiation therapy: Could it be a game-changer for COVID-19? Clin. Transl. Oncol. 2021, 23, 1–4.
- Yazaki, S.; Yoshida, T.; Kojima, Y.; Yagishita, S.; Nakahama, H.; Okinaka, K.; Matsushita, H.; Shiotsuka, M.; Kobayashi, O.; Iwata, S.; et al. Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan. JAMA Oncol. 2021, 7, 1141–1148.
- Bao, R.; Hernandez, K.; Huang, L.; Luke, J.J. ACE2 and TMPRSS2 expression by clinical.; HLA.; immune.; and microbial correlates across 34 human cancers and matched normal tissues: Implications for SARS-CoV-2 COVID-19. J. Immunother. Cancer 2020, 8, e001020.
- Xu, J.; Chu, M.; Zhong, F.; Tan, X.; Tang, G.; Mai, J.; Lai, N.; Guan, C.; Liang, Y.; Liao, G. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 2020, 6, 76.
- Subbarayan, K.; Ulagappan, K.; Wickenhauser, C.; Seliger, B. Expression and Clinical Significance of SARS-CoV-2 Human Targets in Neoplastic and Non-Neoplastic Lung Tissues. Curr. Cancer Drug Targets 2021, 21, 428–442.
- Dai, Y.J.; Hu, F.; Li, H.; Huang, H.Y.; Wang, D.W.; Liang, Y. A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann. Transl. Med. 2020, 8, 481.
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54.
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678.
- Yu, Y.; Wang, M.; Zhang, X.; Li, S.; Lu, Q.; Zeng, H.; Hou, H.; Li, H.; Zhang, M.; Jiang, F.; et al. Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients. Signal Transduct. Target Ther. 2021, 6, 346.
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295.
- Hagemann, K.; Riecken, K.; Jung, J.M.; Hildebrandt, H.; Menzel, S.; Bunders, M.J.; Fehse, B.; Koch-Nolte, F.; Heinrich, F.; Peine, S.; et al. Natural killer cell-mediated ADCC in SARS-CoV-2-infected individuals and vaccine recipients. Eur. J. Immunol. 2022, 52, 1297–1307.
- Di Vito, C.; Calcaterra, F.; Coianiz, N.; Terzoli, S.; Voza, A.; Mikulak, J.; Della Bella, S.; Mavilio, D. Natural Killer Cells in SARS-CoV-2 Infection: Pathophysiology and Therapeutic Implications. Front. Immunol. 2022, 13, 888248.
- Flexman, J.P.; Shellam, G.R.; Mayrhofer, G. Natural cytotoxicity, responsiveness to interferon and morphology of intra-epithelial lymphocytes from the small intestine of the rat. Immunology 1983, 48, 733–741.
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8.
- Li, K.; Methé, B.A.; Fitch, A.; Gentry, H.; Kessinger, C.; Patel, A.; Petraglia, V.; Swamy, P.; Morris, A. Gut and oral microbiota associations with viral mitigation behaviors during the COVID-19 pandemic. Front. Cell Infect. Microbiol. 2022, 12, 966361.
- De Maio, F.; Ianiro, G.; Coppola, G.; Santopaolo, F.; Abbate, V.; Bianco, D.M.; Del Zompo, F.; De Matteis, G.; Leo, M.; Nesci, A.; et al. Improved gut microbiota features after the resolution of SARS-CoV-2 infection. Gut Pathog. 2021, 13, 62.
- Meyer, J.E.; Finnberg, N.K.; Chen, L.; Cvetkovic, D.; Wang, B.; Zhou, L.; Dong, Y.; Hallman, M.A.; Ma, C.C.; El-Deiry, W.S. Tissue TGF-β expression following conventional radiotherapy and pulsed low-dose-rate radiation. Cell Cycle 2017, 16, 1171–1174.
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell Infect. Microbiol. 2021, 10, 596166.
- Ailioaie, L.M.; Litscher, G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. Int. J. Mol. Sci. 2021, 22, 4942.
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu Y Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized.; quadruple-blinded.; placebo-controlled trial. Gut Microbes 2022, 14, 2018899.
- Nayebi, A.; Navashenaq, J.G.; Soleimani, D.; Nachvak, S.M. Probiotic supplementation: A prospective approach in the treatment of COVID-19. Nutr. Health 2022, 28, 163–175.
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral probiotics in coronavirus disease 2019: Connecting the gut-lung axis to viral pathogenesis.; inflammation.; secondary infection and clinical trials. New Microbes New Infect. 2021, 40, 100837.
- Sokol, H.; Contreras, V.; Maisonnasse, P.; Desmons, A.; Delache, B.; Sencio, V.; Machelart, A.; Brisebarre, A.; Humbert, L.; Deryuter, L.; et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 2021, 13, 1–19.
- Scarpellini, E.; Fagoonee, S.; Rinninella, E.; Rasetti, C.; Aquila, I.; Larussa, T.; Ricci, P.; Luzza, F.; Abenavoli, L. Gut Microbiota and Liver Interaction through Immune System Cross-Talk: A Comprehensive Review at the Time of the SARS-CoV-2 Pandemic. J. Clin. Med. 2020, 9, 2488.
- Sencio, V.; Machelart, A.; Robil, C.; Benech, N.; Hoffmann, E.; Galbert, C.; Deryuter, L.; Heumel, S.; Hantute-Ghesquier, A.; Flourens, A.; et al. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes 2022, 14, 2018900.
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G34–G45.
- Zhang, F.; Wan, Y.; Zuo, T.; Yeoh, Y.K.; Liu, Q.; Zhang, L.; Zhan, H.; Lu, W.; Xu, W.; Lui, G.C.Y.; et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients With COVID-19. Gastroenterology 2022, 162, 548–561.e4.
- Howell, M.C.; Green, R.; McGill, A.R.; Dutta, R.; Mohapatra, S.; Mohapatra, S.S. SARS-CoV-2-Induced Gut Microbiome Dysbiosis: Implications for Colorectal Cancer. Cancers 2021, 13, 2676.
- Mozaffari, S.A.; Salehi, A.; Mousavi, E.; Zaman, B.A.; Nassaj, A.E.; Ebrahimzadeh, F.; Nasiri, H.; Valedkarimi, Z.; Adili, A.; Asemani, G.; et al. SARS-CoV-2-associated gut microbiome alteration; A new contributor to colorectal cancer pathogenesis. Pathol. Res. Pract. 2022, 239, 154131.
- Dobranowski, P.A.; Tang, C.; Sauvé, J.P.; Menzies, S.C.; Sly, L.M. Compositional changes to the ileal microbiome precede the onset of spontaneous ileitis in SHIP deficient mice. Gut Microbes 2019, 10, 578–598.
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard, W.A., 3rd; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 2012, 337, 975–980.
- Richter, K.K.; Langberg, C.W.; Sung, C.C.; Hauer-Jensen, M.; Increased transforming growth factor β (TGF-β) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 187-195, .
- Haydont, V.; Mathé, D.; Bourgier, C.; Abdelali, J.; Aigueperse, J.; Bourhis, J.; Vozenin-Brotons, M.C.; Induction of CTGF by TGF-beta1 in normal and radiation enteritis human smooth muscle cells: Smad/Rho balance and therapeutic perspectives. Radiother. Oncol. 2005, 76, 219-225, .
- Ehrhardt, R.O.; Strober, W.; Harriman, G.R. Effect of transforming growth factor (TGF)-beta 1 on IgA isotype expression. TGF-beta 1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J. Immunol. 1992, 148, 3830–3836.
- Wu, M.; Xiao, H.; Liu, G.; Chen, S.; Tan, B.; Ren, W.; Bazer, F.W.; Wu, G.; Yin, Y. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 2016, 60, 1637–1648.
