Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Cancer remains a leading cause of death worldwide, despite many advances in diagnosis and treatment. Precision medicine has been a key area of focus, with research providing insights and progress in helping to lower cancer mortality through better patient stratification for therapies and more precise diagnostic techniques.
- cancer diagnostics
- liquid biopsy
- personalised treatment
1. Introduction
Cancer management remains a major challenge in healthcare, with an estimated 9.6 million cancer-related deaths worldwide and a projected rise of 14–22 million new cases every year [1]. Liquid biopsy (LB) technologies aim to detect cancers at the earliest possible stage to increase survival rates and offer patients personalized treatments for more efficient results. In 2013 and 2016, the first LB tests detecting CTCs (CellSearch® CTC enumeration platform [2]) and ctDNA (cobas EGFR mutation kit v2 [3]), respectively, were FDA approved in the US. Since then, there have been a growing number of approved LB tests for clinical use. Despite these efforts, cancer mortality still proves to be the second-highest cause of death worldwide. The burden of cancer mortality is still incredibly relevant even with the rise of approved LBs. Socioeconomic factors are key in understanding cancer mortality. When investigating mortality rates by ethnicity and income, significant biases can be observed, some of which may result from underfunded healthcare systems, often found in minority communities, thereby leaving individuals without healthcare access and reducing their chances for detection and treatment options. Since the COVID-19 global pandemic had severe implications to healthcare systems and particularly cancer care providers, delays occurred across screening, diagnosis, monitoring, and access to treatments [4]. This has led to an increase in cancer mortality rates and is expected to result in elevated rates in the coming years, due to deferred cancer screening and routine diagnostic check-ups in the UK and the US [4]. In the US, the Centers for Disease Control and Prevention (CDC) reported four times the number of recent projected cancer deaths due to COVID-19 [5]. Studies and public data statistics have shown that there has been a drop between 86% and 94% in preventative cancer screenings for breast, cervical, and colon cancer, due to COVID-19 disruptions [6]. Specifically in the UK, recent findings have reported an increase in mortality rates of up to 10% particularly for breast cancer and 15% for colorectal cancer, based on 5-year survival rates [7]. Decreased hospital screening programs have played a significant role towards this outcome as well as delays across surgical interventions and treatment administration. This phenomenon exposes the importance of affordability and accessibility, emphasizing the need for future postpandemic healthcare models to prioritise improvements in diagnostic technologies.
2. DNA Detection Approaches for Liquid Biopsies
Numerous technologies have been used for research studies and commercially, but most approved methods are based on either traditional qPCR mutational testing, droplet digital PCR (ddPCR), or next-generation sequencing (NGS), as represented in Figure 1.
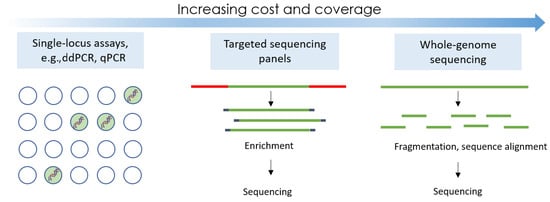
Figure 1. The three main technological principles used in the majority of liquid biopsy assays.
2.1. Traditional PCR-Based Tests
Mutational testing by qPCR has been used in liquid biopsy research studies, with the detection efficacy to rely on the design and optimisation of mutation-specific primers, with the under-study sample to then be analysed and the mutations’ frequencies to be quantified. CancerSEEK is a blood-based PCR analysis system of ctDNA that aims to detect cancer in early stages, with applications in breast, ovarian, lung, liver, colorectal, gastric, and oesophageal cancer [22]. The basis of this test is to detect mutations in 2001 genomic positions, across 16 genes, and assess the levels of eight cancer-associated protein biomarkers, such as HGF (hepatocyte growth factor) and PRL (prolactin) [22], with reported median sensitivity from 73% to 78% and a specificity of more than 99%: 7 out of 812 healthy controls scored positive. Sensitivities varied based on the cancer that was screened for; for instance, breast cancer only showed 33% sensitivity, but 70% sensitivity was depicted for five cancers: ovarian, liver, gastric, pancreas, and oesophageal, for which currently no routine screening procedures are available. The price has been suggested by the team to be less than USD 500 a test. In contrast, the Therascreen PCR kit by Qiagen detects 11 mutations on the individual PIK3CA gene from blood plasma ctDNA and is used to aid in treatment switching instead of early cancer screening. The PIK3 pathway is one the most prevalent and significant oncogenic signalling pathways in breast cancer, present in 70% of breast cancer cases [23]. The presence of mutations in this gene is associated with better patient response to the drug alpelisib (PIQRAY by Novartis), a therapy that aims to inhibit this PIK3 oncogenic pathway [24]. The Therascreen KRAS PCR kit detects 7 mutations (12 ALA, 12 ASP, 12 ARG, 12 CYS, 12 SER, 12 VAL, and 13 ASP) on the KRAS oncogene for metastatic colorectal cancer [25]. The Therascreen test kits cost approximately north of GBP 3000 but also require the use of a Rotor-Gene Q MDX instrument, which costs upwards of GBP 10,000. The Therascreen kits offer a specific use case to be valuable but still require an initial investment for a healthcare setting to perform these tests. Similarly, from both tissue and plasma samples, the Roche Diagnostics cobas v2 test detects 42 mutations in exons 18, 19, 20, and 21 of the EFGR gene, which codes for the tyrosine kinase receptor protein that is reportedly overexpressed in 60% of lung cancers [26,27]. By classifying patients with EGFR mutations, it can offer information to clinicians regarding personalised treatment options. For instance, EGFR tyrosine kinase inhibitors (EGFR-TKIs) are an effective therapy used in non-small cell lung cancer (NSCLC). Patients may develop EGFR tyrosine mutations that void this therapy altogether. The cobas EGFR mutation test v2 costs GBP 125 per test and requires the use of a standard real-time PCR instrument as opposed to Therascreen tests that also require their proprietary RGQ instrument [28].
2.2. Digital Droplet PCR
Digital droplet PCR (ddPCR), the next generation of quantitative PCR (qPCR), divides a sample into many partitions, whereby each partition contains one or two target molecules that undergo individual PCR reactions. This means that ddPCR has higher sensitivity than qPCR; where ddPCR can usually test a mutant abundance of down to 0.1%, qPCR obtains a frequency of approximately 10% [29]. However, ddPCR is more complex and has a higher risk for contamination due to multiple transfer and pipetting steps, while also relying on lengthy workflows executed by specialised scientists. BEAMing, a variant of ddPCR and commercially available as OncoBEAM, is the culmination of bead emulsification amplification and flow cytometry [30]. BEAMing has been a common research practice to study ctDNA because of its increased sensitivity of up to 0.02%, stemming from its ability to separate every DNA molecule in a sample into droplets, forming a massive parallel reaction [9]. OncoBEAM’s robustness and utility has been showcased for numerous cancer types, from the testing of PIK3CA status for metastatic breast cancer with a sensitivity of 81.6% to EGFR mutational status in NSCLC cancer with a sensitivity of 90% [31]. Results also indicated that OncoBEAM plasma results had low minimum allele frequencies with 0.2%, which is below the cobas EGFR mutation test [32]. The turnaround time for OncoBEAM is traditionally 2 days, which is longer than qPCR kits, whereby results are generated on the testing day, but much shorter than NGS, which typically requires a 1-week turnaround time. Overall, OncoBEAM and other ddPCR tests run higher costs than qPCR and require expensive instruments for generating diagnostic results but offer higher sensitivity than qPCR kits and are thus preferred for the analysis of multipanel testing and clinical validation studies [33].
2.3. Next-Generation Sequencing
Sequencing approaches vary from targeted efforts to whole exosomal sequencing (WES) or whole-genome sequencing (WGS) tests. Whilst ddPCR offers great sensitivity, the mutations to screen for must be known in advance, and only a handful of genetic alterations can be studied at a time. NGS is able to bypass this design parameter and screen numerous novel variants. The allele frequency of detection for most sequencing methods is 0.1% [9]. Popular companies that perform or offer sequencers for purchase are Illumina, PACBio, Qiagen, Thermo Fisher, and Agilent. NGS can be applied to targeted panels to increase sensitivity and reduce false positives. Such panels are TAM-Seq, Safe-Seq, and CAPP-Seq [9]. TAM-Seq can identify mutations as low as 2% with sensitivity and specificity over 97% [34]. To reduce error rates, Sysmex’s Safe-Seq utilises unique identifiers that are tagged on all DNA molecules that are analysed, essentially making it a highly specific and sensitive sequencing assay; it has an increased sensitivity and specificity of 98% [34]. Safe-Seq has recently been demonstrated for its use in early breast cancer diagnostics by looking at TP53 and PIK3CA mutations [35]. GRAIL, a company dedicated to creating a platform for pan-cancer early stage screening, has recently begun a large-scale clinical trial using high coverage and breadth sequencing of patient plasma samples to try to create a large ctDNA database [36]. Some newer LB assays also come with companion devices, such as FoundationOne Liquid CDx, which was approved in the US by the FDA to detect over 300 genes with cfDNA using NGS. It has been recently approved to identify patients with BRAF V600E–mutated metastatic colorectal cancer [37]. Its companion device also reports blood tumour mutational burden and microsatellite instability as well as a wealth of other information that can aid in patient stratification and recommend approved therapies for patients [38].
Third-generation sequencing platforms, such as Oxford Nanopore Sequencing [39], combat the accessibility of traditional NGS sequencers by having a technology that can be easily miniaturised, such as the MinION. Nanopore sequencing works by recording electrical changes as DNA molecules pass through a protein nanopore. As each nucleotide passes through the nanopore, a characteristic voltage change is recorded that identifies which nucleotide has just passed through. Due to it working with low DNA input and its ability to sequence long reads of DNA, it has many benefits, such as forgoing PCR in its protocol steps. However, it is not designed for cell-free or fragmented small strands of DNA, such as with a liquid biopsy sample. It is not currently approved in this space; however, much research is still underway for applications in LB diagnostics, especially with copy number variations (CNVs) and methylation profiling.
When comparing costs of NGS tests with those of qPCR or ddPCR, prices vary greatly based on the platform. NGS involves instrument cost, price per run, and price for bioinformatic analysis and, as mentioned, has a larger turnaround time compared with other methods previously mentioned, typically around 1 week. However, the turnaround time for targetted panels is much less than WGS or WES comparatively. Some example prices for instruments are Illumina MiSeq, USD 128k; Ion torrent PGM, USD 80k; PacBio RS, USD 695K; and Illumina HiSeq 2000, USD 654k [40]. Therefore, it becomes a question of what instruments a healthcare setting or research lab has access to, to be able to decide what tests may be feasible for clinicians or researchers to perform. Additionally, simple molecular markers that may not require highly sensitive and quantitative results may prefer to use qPCR kits, whereas tests that require larger genome coverage or in pursuit of novel variants may require NGS.
2.4. CTC Detection Methods
CTCs are valuable for the prognosis and screening of metastatic cancers as low CTC count in blood is correlated with disease-free and overall survival for some metastatic cancers such as breast and colon [41]. The distinctive features of these cells allow their enrichment and detection by various methods. Filters, such as OncoQuick and Ficoll, have been used to capture CTCs based on unique physical properties, while immunological methods have been reported to utilise the expression of surface EpCAM antigens [42].
CellSearch® Menarini Silicon Biosystems (Veritex, Livingston, NJ, USA) is an FDA-approved method that captures CTCs using anti-EpCAM antibodies in a magnetic ferrofluid [43]. The detection relies on the EpCAM antigen’s selective efficacy, which can add a variation in the detection of the CTC presence, which can vary across different stages of disease progression. The 2007 landmark paper for CellSearch® reports an 80% recovery rate detected CTCs in approximately 70% of breast cancer patients. Additionally, the high cost (machinery cost is USD 600–800k) and lengthy sample processing times can make a wide clinical use of this test quite challenging on its current form [44]. Additionally, CellCollector® is a CE-marked system utilising a structured needle with a hydrogel coating containing anti-EpCAM antibodies to bind and isolate EpCAM-positive CTCs after being inserted into patients’ veins. Unlike CellSearch, it does not require the use of expensive instruments, and the isolated CTCs can be further used for molecular analysis and phenotype characterization [45]. CellCollector®, when compared with CellSearch®, showed promise, where in vivo isolation was reported to be 58% of patients positive for at least one CTC compared with 27% for CellSearch® [45]. Whilst being minimally invasive, the assay also requires a complicated enrichment step and a long detection process for the sample (30+ min). Whilst more cost-effective due to not requiring the expensive machinery, it still requires antibodies to run and so has a high run cost [44].
ClearCell FX is another CTC detection system that has been developed for the automated retrieval and enrichment of intact and viable CTCs from a 7.5 ml blood sample. Initial red blood cell lysis is necessary before enrichment, but CTCs remain in suspension, allowing for easy analysis and diagnostics downstream also due to antibody independence. Separation is performed based on the mechanical features of the CTCs, using the spiralised microchannel technology. By eliminating reliance on epithelial cell markers, the ClearCell FX system is able to capture CTCs that do not express the cell markers in addition to cells that have a mesenchymal phenotype, which would be neglected by devices like CellSearch®. Using the ClearCell FX system, CTCs were detected in 100% of peripheral blood samples obtained from breast cancer patients and showed a 50% recovery rate [46]. Furthermore, Parsortix® (Angle PLC, Guildford, UK) has been reported as a system for capturing CTCs from liquid biopsy samples, using an instrument comprising a filtration cassette, with the captured cells to be eluted in a buffer solution for further molecular analysis [47]. They report a 81% recovery rate on whole blood spiked using cultured cell lines.
Lastly, the MagSweeper device was developed to isolate CTCs from liquid biopsy samples through immunomagnetic separation. A magnetic rod, coated in a nonadherent plastic sheath, is robotically swept through a well containing the prelabelled sample. The rod captures the labelled cells and removes them from the whole-blood sample. Through a series of washes and repeated magnetic captures, a sample of CTCs with low contamination is obtained. The CTCs were labelled in the sample using EpCAM-antibody-functionalised paramagnetic beads, which uniquely bind to the target cells, for collection by the magnetic rod. Comparative results from patients diagnosed with metastatic breast cancer and healthy controls identified CTCs in all samples derived from those with cancer and no CTCs in samples from healthy donors [48]. In 2014, Deng et al. used this technology to analyse mutations of the PIK3CA gene in CTCs [49]. The MagSweeper was used to capture and isolate CTCs, and although this technology was successful in obtaining viable cells for further analysis, the reliance of this technology on the presence of EpCAM antigens could lead to events where cells that have undergone EMT could remain undetected. Some examples of certified tests available for each technology type are summarised in Table 1.
Table 1. Summary of key selected liquid biopsy tests and technological platforms presented for the detection of ctDNA and CTCs.
| Testing System | Cancer Type | Technology | Ref. |
|---|---|---|---|
| ctDNA Assays | |||
| Cobas EGFR v2 | Non-small cell lung cancer (NSCLC) | Nondigital, PCR kit | [3] |
| CancerSEEK | Various | qPCR | [22] |
| Therascreen | NSCLC, breast cancer | qPCR | [24] |
| Guardant 360 | Colorectal, breast, NSCLC | NGS | [50] |
| FoundationOne Liquid CDx | Various | NGS | [37,38] |
| GRAIL | Various | NGS | [51] |
| OncoBEAM | NSCLC, colorectal, melanoma | BEAMing (ddPCR) | [52] |
| Precipio | NSCLC | Ice-cold PCR | [53] |
| Freenome | Colorectal, prostate | Multiomics | [54] |
| Oncomine | Lung, breast, and others | NGS | [55] |
| Signatera | Various | NGS | [56] |
| Idylla | Lung, colorectal | PCR | [57] |
| Sysmex Safe-Seq | Breast cancer, head and neck cancer | NGS | [58] |
| CTC Assays | |||
| Cell Search | Breast, prostate, colorectal | Ferrofluid nanoparticles and antibody | [43,59] |
| ClearCell FX1 System | Breast, lung | DFF, microfluidics | [46,60] |
| GILUPI Cell Collector | Lung, colorectal | Anti-EpCam antibodies | [45,61] |
| AccuCyte ®, CyteFinder ® | Prostate, breast, lung | Density-based separation, imaging | [62,63] |
| Parsortix | Various | Microfluidics | [47] |
| OncoQuick; Ficoll | Gastrointestinal, colorectal | Density gradient centrifugation | [64] |
| MagSweeper | Breast, colorectal | Antibodies | [65,66] |
| ImageStream | Hepatocellular carcinoma | Flow cytometry and immunofluorescence | [67] |
2.5. DNA Extraction and Sample Preparation
Despite the method used for ctDNA detection, DNA extraction and sample preparation are vital steps as part of the integration of assays in a system. Sample preparation often consists of four steps: disruption of cells, protein and lipid removal, DNA purification, and then concentration of DNA.
Methods to perform these steps can vary from mechanical approaches, such as ultrasonication, which disrupt cells using pressure, to homogenisation, which shreds cells or chemical methods relying on the use of detergents that break down membranes. Premade extraction kits now exist on the market, simplifying nucleic acid extraction, with examples to include the QIAamp Circulating Nucleic Acid Kit [68] and the Zymo Quick-ccfDNA Serum and Plasma Kit [69], both of which use silica beads to bind DNA molecules, whilst the rest of the sample is washed away. The QIAamp MinElute ccfDNA Midi Kit [70] and the Maxwell RSC ccfDNA Plasma Kit [71] use magnetic beads instead to trap DNA molecules, whilst the rest of the impurities are eluted away. Whether using external extraction kits, silica-column-based precipitation methods, or centrifugation techniques that require in-house equipment, these protocols add complexity, cost, and labour to the utility of nucleic acid detection in cancer care, acting as another barrier to mutational detection for point-of-care testing.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15225434
This entry is offline, you can click here to edit this entry!
