Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
Wound healing is a multifaceted process necessitating the collaboration of numerous elements to mend damaged tissue. Plant and animal-derived natural compounds have been utilized for wound treatment over the centuries, with many scientific investigations examining these compounds. Those with antioxidant, anti-inflammatory, and antibacterial properties are particularly noteworthy, as they target various wound-healing stages to expedite recovery.
- inflammation
- wound healing
- anti-bacterial
- medicinal plants
- Nigella sativa
- natural product
1. Introduction
The Middle East is home to a minimum of 2600 types of plants, with 700 of them cited in Greco-Arab medicinal literature for their healing properties. As per multiple studies, more than 450 of these plants continue to be utilized for human disease treatment and are marketed or exchanged both locally and globally. Out of these plants, 40 are presently utilized for treating a variety of skin issues including acne, psoriasis, and allergies [35,36,37]. Numerous active ingredients have been discovered to be advantageous for the treatment of skin pathologies, as per several in vitro and animal studies. These compounds include β-hydroxychalcone, isoquercetin, ferulic acid, 4-methylthiobutylisothiocyanate, curcumin, sesquiterpene lactone, igalan, linalool, α-peroxyachifolid, quercetin, ursolic acid, saporin, and thymoquinone (Table 1) [13,25].
Table 1. Active compounds with beneficial effects in skin pathology treatments [6,13,24,25,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
| Plant Name | Common English Name | Parts Used | Active Compound |
|---|---|---|---|
| Nigella sativa | Black cumin | Seeds | Thymoquinone |
| Ficus sycomorus | Fig-mulberry | Seeds | Isoquercetin |
| Ferula hermonis | Lebanese viagra | Rhizome and roots | Ferulic acid |
| Aloe vera | Barbadensis Miller | Leaves | Emodin and aloesin |
| Eruca sativa | Arugula | Seeds | 4-methylthiobutylisothiocyanate |
| Curcuma longa | Turmeric | Rhizome | Curcumin |
| Inula helenium | Elecampane | Root | Sesquiterpene Lactone |
| Linum pubescens | Perennial flax | Flower | Igalan |
| Achillea millefolium | Common yarrow | Flower | Linalool, α-peroxyachifolid, stigmasterol and β-sitosterol |
| Urtica dioica | Stinging nettle | Roots | Quercetin and ursolic acid |
| Saponaria officinalis | Soapwort | Roots | Saporin |
| Leptospermum scoparium | Manuka | Aerial parts | β-hydroxychalcone |
| Bletilla striata | Chinese ground orchid | Roots | Coelonin and steroids |
| Rheum officinale | Chinese Rhubarb | Roots | Anthraquinone derivatives |
| Calendula officinalis | Pot marigold | Flowers | Triterpenoids |
| Casearia sylvestris | Crackopen | Leaves | Biciclogermacrene |
| Rosmarinus officinalis | Rosemary | Essential oil | Arnosic acid and rosmarinic acid |
| Crocus sativus | Saffron crocus | Aerial parts | Crocin and safranal |
| Glycyrrhiza glabra | Licorice | Roots and leaves | Glycyrrhizin |
| Hylocereus undatus | Dragon fruit | Fruits | Betacyanins |
| Centella asiatica | Asiatic pennywort | Aerial parts | Asiatic acid |
According to reports, several medicinal plants and their derived active compounds are believed to help hasten the process of wound healing and regenerate tissue at the wound site. Some of the active compounds found in these plants include coelonin, stigmasterol, β sitosterol, anthraquinone derivatives, triterpenoids, rosmarinic acid, crocin, safranal, curcumin, emodin, aloesin, betacyanins, Asiatic acid, thymoquinone, and thymol (Figure 1) [6,24,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
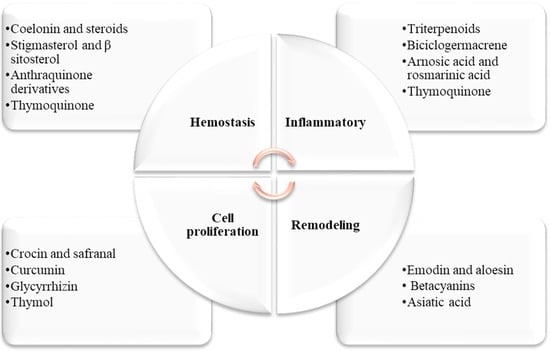
Figure 1. Dermatological herbal active compounds and their effects on the four phases of wound healing.
2. Traditional Uses and Active Compounds of N. sativa
N. sativa, also known as black cumin or black seed, is an herbaceous plant belonging to the Ranunculaceae family. It produces an annual flower with five to 10 petals that can be white, yellow, pink, light blue, or lavender in color. The plant’s fruits are large swollen capsules that contain three-dimensional white seeds. These seeds turn black after being exposed to air and have a fragrant and bitter flavor [53,54,55]. For many centuries, N. sativa has played a significant role in the culture, cuisine, and traditional medicine of South Asia, as well as in Europe and the Mediterranean region (Figure 2) [56].
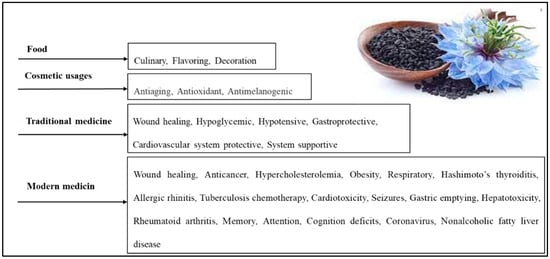
Figure 2. Various N. sativa seed applications [57].
N. sativa is widely known and used in the Arab world, where it is called kalonji and Habbatul Barakah, meaning “seed of blessing” in Arabic. The seeds are often used as an ingredient in bread and pastries, and a dessert made from it is prepared by mixing it with flour and roasting it in the oven (Figure 2) [13,57]. Historical records from various scientific and religious texts have documented the curative properties of N. sativa. According to Islamic Hadith, the Prophet Muhammad instructed others to “use the black seed, for without a doubt, it is a cure for all sicknesses except death.” Avicenna (980-1037 AD), an ancient Muslim physician and philosopher, mentioned N. sativa in his famous “Canon of Medicine”, which was part of the traditional medical curriculum from the 12th to the 17th centuries [58,59]. In his canon of medicine, Avicenna described the seeds as “the seed that enhances the energy production in the body and facilitates recovery from fatigue and dejection”, weariness, fever, headache, skin ailments, wounds, fungus, parasites, and deadly animal attacks [59,60,61,62,63].
The principal constituents of N. sativa seeds are fixed oil—33.3%, proteins—23.45%, carbohydrates—31.8%, crude fiber—7.2%, minerals—3.3%, and essential oil—0.95%. The therapeutic effects of the seeds are attributed to the main active compounds thymoquinone, dithymquinone, thymohydroquinone, and thymol. Other chemicals found in the seeds comprise nutritious components including carbohydrates, lipids, vitamins, minerals, and vital amino acids. They contain a high concentration of essential and unsaturated fatty acids such as linoleic acid and oleic acid. Phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatitdylinisitol are also found in the seeds. The seeds also include calcium, iron, potassium, and carotene, which the liver converts into vitamin A [64,65].
The surge in interest in phytomedicine has raised concerns about their safety and the legal obligations to comply with health standards [66]. N. sativa is “generally recognized as safe” by the FDA in the United States and is available in various therapeutic formulations such as dietary supplements, oils, topical creams, and powders [56]. Numerous clinical trials and research have been conducted to examine its effects on different medical conditions, with evidence indicating tolerable and effective dosages of roughly 1–3 g of powder once day, 40 mg/kg of oil once daily, or 1 mL of topical cream three times per day [67,68]. A topical administration of N. sativa cream, oil, or hydrogel is optimal for cutaneous dermatological problems, whereas oral oil or supplement formulations are suitable for systemic dermatological disorders. Although no significant negative effects of the oral oil supplements, topical cream, or hydrogel formulations have been observed, three women who used the topical oil formulation had acute contact dermatitis [56,67,68]. A recent phase 1 trial—which was randomized, double blinded, and placebo controlled—assessed the safety of a N. sativa oil formulation containing 5% TQ (BCO-5). The trial administered a dose of 200 mg/adult/day for 90 days to healthy subjects (n = 70). The study analyzed both biochemical and hematological parameters, along with any adverse events or side effects, to determine the clinical safety of BCO-5. The study found no serious adverse side effects or significant changes in the hematological parameters. Similarly, no significant changes were observed in the liver function (ALT, AST, ALP) and renal function (serum creatinine and urea). However, a significant reduction in total cholesterol, LDL, VLDL, and triglycerides was noted in the lipid profile analysis, although these values remained within the normal range. Therefore, BCO-5 should be clinically evaluated for various health beneficial pharmacological activities at a dosage of 200 mg/adult/day [69,70].
The dermatological benefits of N. sativa and thymoquinone are attributed to their potent antioxidant, anti-inflammatory, antibacterial, and immunomodulatory properties, making them suitable choices for the treatment of several skin diseases (Figure 2). Nasiri et al. [71] conducted a meta-analysis of 14 randomized controlled trials to evaluate the effectiveness of N. sativa products for treating various skin conditions. The participants of these trials had different types of skin diseases, such as psoriasis, eczema, and acne. The average age of the participants was 28.86 (4.49), and 69% of them were female. The duration of the treatment ranged from 4 days to 24 weeks. A meta-analysis showed that N. sativa products had a significant effect on improving the symptoms of skin diseases, with an odds ratio of 4.59. Most of the trials used N. sativa essential oil or extract, applied either orally or topically. The researchers concluded that N. sativa products could be used as an alternative therapy for skin problems, but more research is needed to confirm their efficacy and safety.
3. The Beneficial Wound-Healing Effects of Thymoquinone
N. sativa and thymoquinone have been shown to have a wide range of therapeutic benefits (Figure 3). These characteristics, which include anti-inflammatory, anti-allergic, antioxidant, anticancer, and antibacterial properties among others, have been proven through a variety of in vitro and animal studies [8,57,72]. The positive effects of thymoquinone on skin wound healing are primarily due to the induction of angiogenesis, anti-inflammatory, antioxidant, and antibacterial effects, as well as an increase in fibroblast proliferation and collagen synthesis. These processes work together to promote the healing of skin wounds (Figure 3 and Figure 4) [24,25].
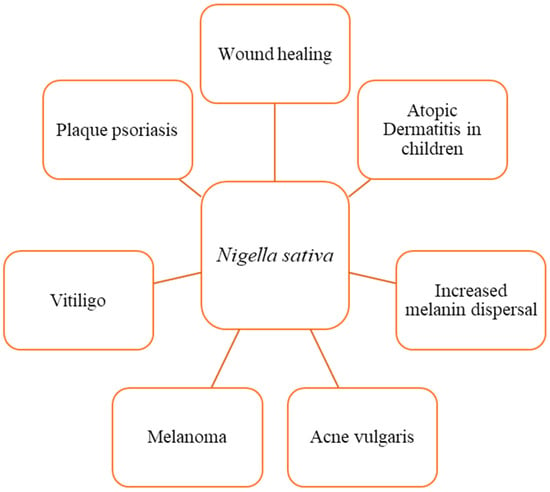
Figure 3. Dermatological effects of N. sativa and its active compound, thymoquinone. Several in vitro and animal studies have recognized N. sativa and thymoquinone for their extensive therapeutic beneficial effects, which stem from their anti-inflammatory, anti-allergic, anti-diabetic, antioxidant, anticancer, antibacterial, nephroprotective, and neuroprotective characteristics.
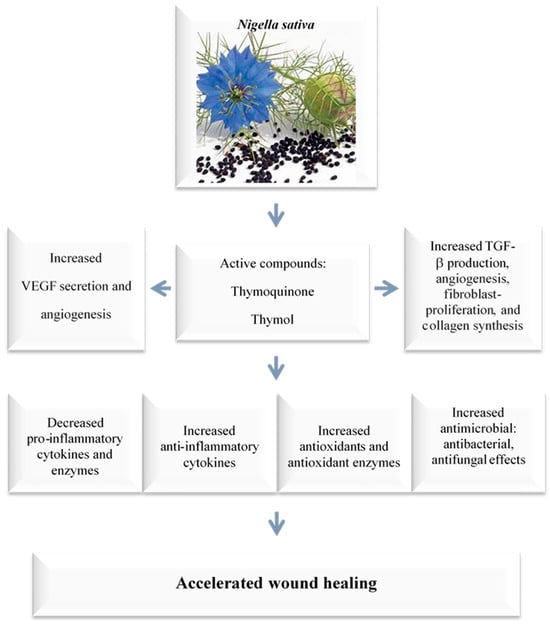
Figure 4. Wound-healing mechanisms of N. sativa and thymoquinone. The positive effects of thymoquinone on skin wound healing primarily stem from its antibacterial effects; the induction of angiogenesis, anti-inflammatory, and antioxidant properties; as well as an increase in fibroblast proliferation and collagen synthesis. These processes collectively promote the healing of skin wounds. Vascular endothelial growth factor (VEGF); Transforming growth factor beta (TGF-β).
Thymoquinone has been shown to significantly reduce the tissue damage caused by ischemia-reperfusion, a condition in which blood flow is temporarily restricted and then restored to an organ or tissue [73]. In addition, thymoquinone has also been shown to have antibacterial and resistance-modifying effects against a variety of infections [4,5]. It has also been shown to boost anti-inflammatory effects, reduce oxidative stress, enhance fibroblast proliferation, increase granular tissue growth and development, speed up wound contraction, and improve re-epithelization [44,74,75,76]. Selcuk et al. [74] investigated the wound-healing activity of thymoquinone in a rat model and concluded that thymoquinone’s wound-healing activities are due to its anti-microbial, resistance-modifying, antioxidant, and anti-inflammatory effects [44,72,73,74,75,76]. Applying thymoquinone topically to wounds is an effective approach because it allows for direct penetration to the affected areas [77]. However, the therapeutic effectiveness of thymoquinone is limited since it has low water solubility and low skin penetrability, as well as restricted systemic availability. Despite these limitations, thymoquinone has shown promise for use in medical applications due to its multifunctional activity [78,79].
In a study examining the safety of thymoquinone for patients with advanced refractory malignant disease, it was found that thymoquinone was well tolerated in doses ranging from 75 mg/day to 2600 mg/day. The study did not report any toxicities or therapeutic responses [80].
3.1. Thymoquinone’s Effects on Inflammation and Immune System Modulation
Immunomodulation is the alteration of the body’s innate and adaptive immune responses, which involves managing the communication between different components like neutrophils, macrophages, and T and B-cells. Immunomodulators, which can either stimulate or suppress the immune system through cellular interactions and paracrine and autocrine mechanisms, play a crucial role in supporting immune function [81].
The innate immune system is made up of numerous protective strategies that act as the initial barrier against potential threats. Cells within this system produce pro-inflammatory cytokines, which boost the body’s defense mechanisms by generating antimicrobial mediators, the migration of leukocytes, and creating a protective environment that shields tissues from microbial invasions. During the later stages of the inflammatory phase in the wound-healing process, it has been observed that macrophages transition from a pro-inflammatory to an anti-inflammatory phenotype. The innate immune system is capable of rapidly activating and launching responses to harmful agents and their products, which can range from pathogen-associated molecular patterns (PAMPs) to damage-associated molecular patterns (DAMPs). The immune system’s pattern recognition receptors, including Toll-like receptors (TLR), identify these unique patterns, thereby triggering cellular defenses. These defenses include pro-inflammatory cascades against internal or external danger signals, foreign entities such as viruses and bacteria, and particles. For a more detailed review of innate immunity in chronic wounds, please refer to the following section [82,83].
In essence, inflammation is a crucial, nonspecific response of the innate immune system. This innate immune system not only interacts with but also guides and instructs the adaptive immune system to produce the most effective immune responses [84]. In addition, the reaction of the innate immune system is closely linked to the production of immune-mediators such as interferons (IFNs), interleukins, and antimicrobial peptides and proteins (AMPs), along with acute-phase proteins. The innate immune system comprises macrophages, neutrophils, mast cells, eosinophils, and innate lymphoid cells. The subsequent subsections provide a more detailed explanation of these cells’ role in wound healing. Figure 2 illustrates the different immune cells involved and their respective roles and interconnections in the wound-healing process [84].
The immunomodulatory properties of thymoquinone have been a significant area of research for many decades, with the goal of enhancing the human immune system [85,86]. Investigations into the immunomodulatory effects of thymoquinone have been conducted in both animal studies and under in vitro conditions. These studies have shown that thymoquinone has the ability to control the development and cellular reactions of various immune cells, such as T-cells, B-cells, macrophages, neutrophils, NK cells, and dendritic cells [87]. Research on thymoquinone has indicated its potential effects on gestational diabetic females. It has been suggested that thymoquinone can reverse the reduced production of interleukin-2 (IL-2) and enhance the production of T-cells, thereby maintaining T-cell-mediated immune responses [85]. In another study, it was found that a small amount of thymoquinone can enhance the survival of T-cells during CD85 activation and CD62L expression. This led to the conclusion that thymoquinone could be a potent agent in combating infectious diseases. Additionally, it was noted that thymoquinone could enhance T-cell activation, thereby improving adaptive immunity [85]. Moreover, there is compelling evidence that thymoquinone has a modulatory effect on the nuclear factor erythroid 2-related factor 2 (Nrf2). This effect is reported to occur through the inhibition of NF-κB signaling pathways [86].
The role of thymoquinone in innate immunity is well defined, as it has been demonstrated to inhibit the maturation of dendritic cells induced by lipopolysaccharides. This is achieved through the activation of the caspase cycle, along with various interleukins and TNF-α [86]. Moreover, thymoquinone has the ability to halt and reverse the reduction in leukocyte count and immunoglobulin levels caused by pesticides, as it enhances phagocytic activation by stimulating macrophages. These studies highlight the inherent characteristics of thymoquinone as an immunomodulatory agent, capable of effectively stimulating innate immunity through the activation of various immune cells. While these reports do not directly link thymoquinone to anticancer immunity, they do successfully underscore the immunomodulatory properties of thymoquinone and its potential as an immunotherapy agent [85,86,87,88,89].
With the aim of elucidating the mechanisms of the anti-inflammatory and antioxidative activities of thymoquinone, Kundu et al. [90] conducted research to clarify the anti-inflammatory and antioxidative effects of thymoquinone at the molecular level. They discovered that when hairless mouse skin was pretreated with thymoquinone, it reduced the expression of COX-2 induced by 12-O-tetradecanoylphorbol-13-acetate (TPA). Moreover, it was discovered that thymoquinone can lessen the nuclear translocation and DNA binding of NF-κB by inhibiting the phosphorylation and subsequent degradation of IκBα in TPA-treated mouse skin. Thymoquinone pretreatment also reduced the phosphorylation of Akt, c-Jun-N-terminal kinase, and p38 mitogen-activated protein kinase but not extracellular signal-regulated kinase-1/2. The topical application of thymoquinone led to an increase in the expression of heme oxygenase-1, NAD(P)H-quinoneoxidoreductase-1, glutathione-S-transferase, and glutamate cysteine ligase in mouse skin. The researchers inferred that thymoquinone’s capacity to suppress TPA-induced COX-2 expression and NF-κB activation, along with its ability to stimulate the expression of cytoprotective proteins, forms the mechanistic foundation for its anti-inflammatory and antioxidative effects (Figure 4).
In recent research conducted by Hijazy et al. [91], the effects of oral administration on the development of edema, oxidative stress, and inflammation in mice that had paw edema were investigated. The study found that thymoquinone reduced paw edema volume over time, lessened writhing movements caused by acetic acid, and decreased ear edema triggered by xylene. Hematological results showed the significant normalization of altered WBC and platelet counts. Additionally, paw tissue levels of malondialdehyde and nitric oxide decreased significantly, while Nrf2, glutathione, superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase increased after thymoquinone administration. Thymoquinone has been shown to reduce pro-inflammatory mediators in inflamed paw tissue, including IL-1, TNF-α, IL-6, MCP-1, C-reactive protein, myeloperoxidase, and NF-κB. In addition, thymoquinone treatment in mice resulted in significant decreases in cyclooxygenase-2 and its product prostaglandin E2, as well as the immune reaction of TNF-α. Histopathological analysis further confirmed the antiedematous and anti-inflammatory effects of thymoquinone in inflamed tissues. The researchers concluded that the results support the potential use of thymoquinone to alleviate acute inflammation due to its strong antioxidant and anti-inflammatory properties in inflamed paw tissue.
3.2. N. sativa and Thymoquinone’s Antibacterial Properties
The healing of wounds can be delayed by infections and pathological conditions such as cellular disorders, ischemia, neuropathy, and angiogenesis. This is especially true for diabetic patients. Recent research has improved our understanding of the association between wound healing and the skin microbiome [92]. The interactions between different species within the microbial environment are dynamic and can change bacterial behavior, resulting in increased virulence and delayed wound healing. A balanced or diverse microbiome, on the other hand, is necessary for effective wound healing because it inhibits pathogen development, lowering the risk of infection, persistent inflammation, and chronic wounds. Due to the complexity and dynamic nature of the skin wound-healing process, it is necessary to use combined treatments that target both the host and the microbiome. Encouraging findings on skin microbiome modifications in wound healing and dysbiosis-related skin disorders point to novel treatment options [93]. However, further studies are needed to better understand how the skin microbiome collaborates with the host during wound healing.
In this regard, a large amount of published data suggests that the constituents of N. sativa seeds have the potential to modulate the immune system, which could affect the relationship between the host and parasites. In line with this, it has been reported that the active compounds of N. sativa oil and seeds have antimicrobial properties, including antibacterial, antifungal, anthelminthic, and antiviral effects [63]. It has been suggested that some of the antimicrobial effects of N. sativa seeds are due to the immunomodulatory properties of their components. N. sativa has been found to have antibacterial activity against several strains of bacteria, including Escherichia coli (E. coli), Bacillus subtilis (B. subtilis), Streptococcus faecalis (S. faecalis), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa). It also has antifungal properties and has been shown to be effective against the pathogenic yeast Candida albicans (C. albicans) and other fungi [48,94,95]. In a previous study, dithymoquinone was found to have antibacterial effects against Gram-positive bacteria [95]. Additionally, a diethyl ether extract of N. sativa was shown to inhibit the growth of Gram-positive bacteria S. aureus, as well as Gram-negative bacteria P. aeruginosa and E. coli, in a concentration-dependent manner. Notably, the extract was more effective against drug-resistant bacteria, including Vibrio cholera (V. cholera), E. coli, and all strains of Shigella dysentriae (S. dysentriae) [48]. The in vivo antibactericidal effectiveness of N. sativa seed constituents might be influenced by a variety of host conditions. When mice are injected with C. albicans, colonies of the fungus grow in various organs. Using this paradigm, a study of the anti-fungal impact of aqueous extract of N. sativa seeds revealed that treating infected mice daily for three days, beginning 24 h after C. albicans inoculation, significantly suppressed fungus development in the liver, spleen, and kidneys [94].
In individuals with diabetes, wound recovery can be significantly hindered by microbial infections. Khan et al. [94] tested a salve made from ostrich oil, honey, beeswax, extracts of N. sativa, propolis, and Cassia angustifolia on the wound-healing process in diabetic rats, and the results were encouraging. The results showed that the ointment had remarkable antibacterial activity against common bacteria such as S. aureus, E. coli, Acinetobacter baumannii, and P. aeruginosa. When compared to the control group, the ointment considerably accelerated wound healing and boosted collagen deposition in vivo. A histopathological evaluation also revealed the presence of hair follicles, sebaceous glands, and vessels in the group treated with the ointment. These results suggest that the ointment was successful in rapidly healing diabetic wounds and could be a suitable candidate for wound healing [94].
A recent study examined the physicochemical effects of N. sativa honey from the Burdur region of Turkey, as well as its antioxidant and antimicrobial properties. The honey samples showed high antimicrobial activity against several bacteria, including S. aureus, E. coli, Chromobacterium violaceum, Bacillus cereus, Klebsiella pneumoniae, and Acinetobacter haemolyticus [96].
The evidence discussed above demonstrates that the constituents of N. sativa seeds have anti-microbial properties against a variety of pathogens, including bacteria, viruses, helminths, and fungi. This is extremely important in practice since N. sativa seeds have been used historically and therapeutically in the Arab and Islamic world with no recorded harmful effects. In essence, it might be a useful co-therapeutic agent against a variety of bacteria. Additional research is needed, however, to understand the particular mechanisms of N. sativa’s antimicrobial properties, both alone and in combination with pharmaceuticals, and to evaluate its potential therapeutic effects on other bacterial, viral, and parasitic models [97].
3.3. The Protective Impacts of N. sativa and Thymoquinone against Oxidative Damage
Oxidation is a chemical process that can damage cells and tissues by producing harmful molecules called reactive oxygen species (ROS). Antioxidants are substances that can stop or slow down oxidation by neutralizing or removing ROS, even when they are present in small amounts [98,99]. Antioxidants, substances that can stabilize or scavenge free radicals, can be divided into two categories: natural and synthetic. Natural antioxidants are obtained from sources such as food and plants and are widely available. These include polyphenols, carotenoids, and vitamins derived from plant materials. Synthetic antioxidants, by contrast, are artificially created and do not come from natural sources [99,100]. Fruits, vegetables, nuts, seeds, leaves, roots, and bark are abundant in antioxidants and are packed with natural compounds that aid in cell protection against free radical damage [101]. In addition to their fundamental antioxidant properties, naturally occurring antioxidants have a wide range of biological functions and provide significant nutritional benefits. They are completely safe to eat and have no known adverse effects. These antioxidants have several health advantages and can help protect cells from free radical damage.
In recent years, there has been growing interest in the use of plant-derived products for managing and treating wounds. Plant leaves, fruits, and seeds are thought to be potential antioxidant sources. Because of their quick development and abundance, leaves are one of the most important sources of antioxidants. Fruits and seeds are also well regarded for their antioxidant capacity due to their phytochemical properties and nutritional contents such as fibers, vitamins, and minerals [102].
At low concentrations, ROS can safeguard tissues from infections and promote efficient wound recovery by triggering signals for cell survival. [103]. However, when present in excess, ROS can induce cell damage and a pro-inflammatory state, leading to oxidative stress [104,105]. Polyphenols, for example, are antioxidants that can transfer electrons to other molecules, including ROS. This prevents electrons from being sequestered from other physiologically vital molecules such as proteins or DNA. Furthermore, antioxidants can initiate an intricate series of events that convert ROS into more stable compounds. As a result, these compounds contribute to the maintenance of non-toxic levels of ROS in wound tissues, which can aid in the healing process [45,106].
The anti-inflammatory, antioxidant, and antibacterial properties of N. sativa and its active constituent, thymoquinone [81], were found in many scientific reports to accelerate wound healing (Figure 4). For example, Bordoni et al. [107] evaluated N. sativa oil′s antioxidant properties in inflamed adipose tissue using human preadipocytes. The total antioxidant activity measured in the supernatant of the preadipocytes showed that N. sativa oil has very high residual activity. Tiji et al. [108] compared the antioxidant effects of different extracts and fractions obtained from N. sativa seeds using hexane and acetone as solvents. They found that the extracts contained fractions with varying levels of antioxidant activity, which might be related to the presence of specific secondary metabolites in each fraction, such as polyphenols. This result is consistent with another study that reported that the methanolic extract of N. sativa seeds had higher antioxidant activity than the aqueous extract [109]. Ouattar et al. [110] investigated the antiradical activity of N. sativa extracts using different solvents and doses. They found that the crude extract had lower antiradical activity than the n-butanol or ethyl acetate extracts. These results suggest that N. sativa has antioxidant properties, which have been confirmed by various methods in other studies. However, the level of antioxidant activity depends on the type of extract and the fraction tested [111].
This entry is adapted from the peer-reviewed paper 10.3390/cimb45110567
This entry is offline, you can click here to edit this entry!
