Amoebae found in aquatic and terrestrial environments encompass various pathogenic species, including the parasite Entamoeba histolytica and the free-living Acanthamoeba castellanii. Both microorganisms pose significant threats to public health, capable of inducing life-threatening effects on humans. These amoebae exist in two cellular forms: trophozoites and cysts. The trophozoite stage is the form used for growth and reproduction while the cyst stage is the resistant and disseminating form. Cysts occur after cellular metabolism slowdown due to nutritional deprivation or the appearance of environmental conditions unfavourable to the amoebae’s growth and division. The initiation of encystation is accompanied by the activation of stress responses, and scarce data indicate that encystation shares factors and mechanisms identified in stress responses occurring in trophozoites exposed to toxic compounds derived from human immune defence.
1. Introduction
Protists of the Amoebozoa clade include several human pathogens, such as
Entamoeba histolytica and
Acanthamoeba castellanii, which significantly impact human health. They grow and reproduce in an “active” form called a trophozoite. When subjected to stressful conditions, such as nutrient deprivation, they can efficiently protect themselves by forming cysts in a mechanism called encystation [
1,
2]. Cysts are characterized by cellular quiescence. Their formation ensures the survival of the species because they resist environmental changes and are easily transmitted to hosts. This is the main contaminating form of these amoebae. Due to their high resilience, cysts also protect from the hosts’ immune system and from biocide treatment.
The free-living
Acanthamoeba castellanii is found in the environment where it feeds on other microorganisms, such as bacteria, which in turn can use
A. castellanii as a host or even a vector. The microorganisms found within
A. castellanii are de facto protected from environmental stresses such as biocides. This way, the amoeba plays a significant role in hosting and disseminating other human pathogens [
3]. Thus, in the context of global warming,
A. castellanii represents a concern for the emergence of unknown pathogens (e.g., giant viruses revived from prehistoric permafrost [
4]).
A. castellanii is also a human pathogen
per se since it provokes amoebic keratitis, blinding, and granulomatous amoebic encephalitis [
3,
5]. There are still no specific drugs to treat these diseases [
6]. For anti-
A. castellanii treatments, clinicians use biguanides such as chlorhexidine and polyhexamethylene biguanide, which impair the integrity of cell walls [
7,
8]; diamidines such as propamidine isethionate which inhibit DNA synthesis [
9]; an alkylphosphocholine like miltefosine that seems to have pleiotropic effects [
10]; or the cytotoxic agent hydrogen peroxide [
11]. These molecules are not always effective, due to the persistence of the cystic stage, and recurrences may occur.
Entamoeba histolytica, responsible for amoebiasis, is a eukaryote devoid of mitochondria and a strict parasite of humans. Its cysts contaminate nearly 20% of the population in suburban and rural areas of countries where sanitation practices are compromised. The trophozoite derived from excystation resides in the intestinal lumen where it feeds on bacteria from the microbiota. Within the human gut, this amoeba converts from avirulent to virulent and destroys the muco-epithelial barrier, leading to overproduction of mucus, the killing of human cells, inflammation, and dysentery [
12]. The highly mobile trophozoite faces various stresses derived from the host immune response, including oxidative and nitrosative stresses that constitute a challenge for amoebic survival. No vaccine against amoebiasis currently exists. The drug of choice for therapy is metronidazole, which may cause severe side effects in humans and on anaerobic members of the microbiota [
13]. The world is widely unprepared for an outbreak of
E. histolytica due to the lack of a vaccine.
Amoebae submitted to nutrient starvation trigger stress responses [
2,
13] equivalent to protective responses activated when they are exposed to toxic compounds derived from human immune defences. Omics data show that the regulation of protein abundance (i.e., Hsp20 in
A. castellanii or Hsp70, chitinase, and cyst wall proteins in
E. histolytica) [
14,
15] and upregulation of gene expression by RNAs (i.e., tRNA, anti-sense RNA, and mRNA) are common to encystation and oxidative stress [
1,
16,
17,
18,
19]. Despite the importance of pathogenic amoebae in ecology and medicine, the stress responses of
Acanthamoeba spp. and
Entamoeba spp. remain under-investigated.
2. Mechanisms of Stress in Pathogenic Amoebae during Encystation
Encystation is a differentiation process triggered in response to prolonged nutritional deprivation stress. From the trophozoite state, the pathogenic amoebae differentiate into dormant resistant cysts. The unpublished data indicate that early morphology changes leading to encystation can be followed in living trophozoites using video microscopy, as shown in
Figure 1 with
A. castellanii. This process requires the total reshaping of trophozoites that become rounded. It is known that cyst-wall components are then synthesized [
29] and transported toward the plasma membrane in specific encystation vesicles containing fibrillar material [
30,
31,
32]. The membrane trafficking system is also modulated since secretory pathway- and trans-Golgi network-endosome-related genes are upregulated during cyst formation [
33]. Gradual dehydration reduces trophozoite volume by approximately 80% following retraction of the cytoplasm from the cyst wall [
34]. Cell signalling under encystation stress increases cAMP levels and activates protein kinase A, known to widely control amoebozoan encystation. At the biochemical level, glycogen is rapidly degraded for cyst-wall synthesis. Sugar polymers that make fibrils include beta-1,4-linked GlcNAc (chitin) and beta-1,4-linked glucose (cellulose).
Entamoeba makes chitin, while the
Acanthamoeba cyst wall is mainly composed of cellulose. AMPK, the enzyme that maintains ATP homeostasis [
23], has been shown to be involved in encystation, as well as in heat stress and OS of
Entamoeba [
35], and has homologs in
Acanthamoeba that could also be involved in stress responses.
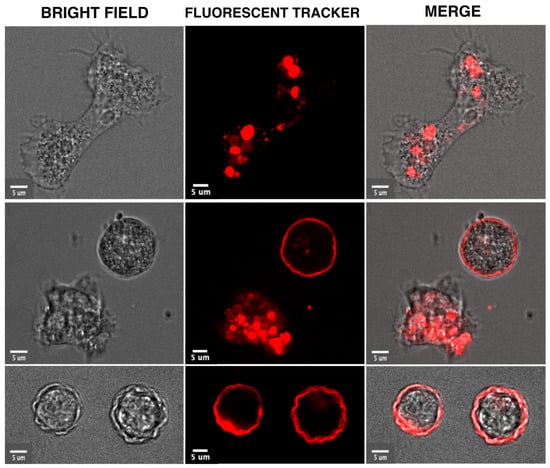
Figure 1. Morphological and intracellular changes visualized in video microscopies acquired during the encystation of
A. castellanii. The trophozoites were incubated in media containing a red fluorescent tracer (Cell Tracker™ Red) which accumulates in cytoplasm and intracellular compartments. The unpublished micrographs show the elongated trophozoite in a rich medium (upper panel) and a rounded trophozoite stressed by food deprivation next to a cyst (middle panel) and cysts (third panel). These cellular forms were visualized by confocal microscopy with bright field (left panel) or fluorescence light (middle panel). The two micrographs are overlaid in the right panel. Bar corresponds to 5 μm.
3. Mechanisms of Stress in Pathogenic Amoebae during Accumulation of Reactive Oxygen Species
Increase of ROS levels (i.e., peroxides, superoxides, hydroxyl radicals, and singlet oxygen), play important roles in cell signalling and homeostasis, and cause damage to the cells through Oxidative Stress (OS). Mitochondria-containing amoebae (e.g.,
Acanthamoeba) react to ROS (as other eukaryotes) via oxidative phosphorylation through the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase [
36]. However, high levels of ROS damage the membrane structure by targeting lipids and proteins. The literature reports several molecules able to regulate the
Acanthamoeba OS response. Exposition to pure oxygen has been shown to increase production in ROS [
37], whereas chlorhexidine increases the ROS levels in
Acanthamoeba through a reduction in the activity of antioxidant enzymes such as the superoxide dismutase (SOD) and the flavin adenine dinucleotide–fumarate reductase (NADH-FRD) [
38].
Amitochondriate amoebae (e.g.,
Entamoeba) respond to OS using a large arsenal of redox pathways [
44], since the basic antioxidant defence mechanisms of eukaryote cells (such as reduced glutathione, catalase, and glutathione-recycling enzymes) lack in
Entamoeba and their major antioxidant is L-cysteine, a sulphur-containing amino acid [
45]. In the absence of mitochondria,
Entamoeba species retain an organelle derived from mitochondria called mitosome devoid of any organellar genome. It is the site of sulfolipid synthesis, and certain enzymes of this pathway are involved in encystation [
46,
47]. The mitochondrial eukaryote iron–sulphur assembly system is replaced in
E. histolytica by a nitrogen fixation system whose coding genes were acquired through lateral gene transfer from ε-proteobacteria [
48]. Iron–sulphur (Fe–S) clusters are ubiquitous cofactors that mediate a multitude of functions including electron transfer and redox reactions. In mitosomes, the
Entamoeba hydroxyperoxyde detoxification enzyme rubrerythrin contains Fe-S clusters [
49]. Whereas the eukaryotic assembly of Fe-S cofactors is mediated by proteins inherited from the original mitochondrial endosymbiont, in
E. histolytica, the analysis of two genes encoding critical Fe-S cluster (Isc) biosynthetic proteins suggests a lateral acquisition from epsilon proteobacteria [
48,
49,
50]
4. UPR and Endoplasmic Reticulum Stress Responses Identified in Pathogenic Amoebae
Entamoeba histolytica maintain intracellular hypoxia in human oxygenated tissues and cellular homeostasis during the host immune defence attack. The trophozoites stimulate cysteine synthase activity, inhibit glycolysis, and induce an Unfolded Protein Response (UPR) [
55]. Considering that no classic ER structure has been described in
E. histolytica, studies regarding UPR have remained scarce. In mammalian cells, UPR is activated in parallel by three ER transmembrane sensors: the inositol-requiring enzyme 1 (IRE1), the protein kinase RNA-like ER kinase (PERK), and the activating transcription factor 6 (ATF6). This leads to an increase in the transcription of chaperone-encoding genes, e.g., Hsp70, whose over-expression attenuates the UPR. In
E. histolytica, UPR- and ER-associated degradation are interconnected, as shown by the upregulation of Ubc7 and IRE1 upon the accumulation of misfolded proteins in the ER [
56]. PERK is activated by misfolded proteins and phosphorylates the alpha subunit of eukaryotic initiation factor-2 (eIF2α), causing a reduction in protein synthesis and the preferential translation of mRNA coding for from stress-related genes.
E. histolytica’s genome does not encode obvious orthologues of PERK or ATF6; however, the phosphorylation of eIF2α occurs after serum starvation, heat shock, OS [
57], and ER stress [
58]. In
E. invadens, silencing the gene encoding EieIF2α (homologous with eIF2α) leads to increased sensitivity to OS and a reduction in encystation rate [
59]. These data suggest that there could be an incomplete UPR pathway in
Entamoebae [
57]. Virulent
E. histolytica cultured under OS display massive upregulation of genes encoding HSPs [
13] and diverse transcriptome analysis of
E. histolytica, highlighting that the Hsp70-A-encoding gene is upregulated following OS and nitrosative stress (NO), and during the formation of hepatic abscesses; therefore, the inhibition of Hsp70-A blocks virulence [
60]. The treatment of
E. histolytica with NO inhibits glycolysis, enhances cysteine synthase activity, and dramatically provokes extensive ER fragmentation [
61] (
Figure 2). As in other systems [
62], ER stress and OS in
Entamoebae seem to be strongly correlated, although the above-described specificities give perspectives for new drug discovery.
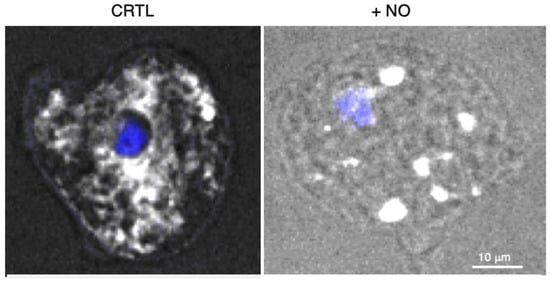
Figure 2. E. histolytica submitted to stress by the addition of nitric oxide (NO). The endoplasmic reticulum was followed by confocal microscopy, recovering the fluorescence of the green fluorescent protein-FLAG construct linked to the ER retention motif KDEL (white signal obtained with an anti-FLAG antibody) and the nucleus (4′,6-diamidino-2-phenylindole, DAPI in blue). Protocol details are in reference [
58]. The fluorescence is homogenous in resting condition (left panel), whereas ER fission is observed as vesicular patterns in the presence of NO (right panel). Bar scale corresponds to 10 μm.
5. Ubiquitin, Ubiquitin-like Protein Modifications, and Proteasome Activities in Pathogenic Amoebae
Stress leads to ubiquitin and ubiquitin-like protein modifications that target protein degradation by the proteasome. Finely regulated physiological processes rely on post-translational protein modification with ubiquitin (Ub) and ubiquitination constitutes a major source of proteome regulation during stresses. The covalent attachment of Ub (a 76-residue peptide) is called monoubiquitination and can be followed by the addition of more Ub: multiubiquitination or polyubiquitination of proteins. The degree of ubiquitination accounts for diverse regulations of the substrate. For example, polyubiquitinated proteins are typically degraded by the 26S proteasomal complex, whereas mono/multiubiquitinated proteins are not degraded and can be involved in endocytosis, membrane trafficking, regulation of kinase signalling, DNA repair, and chromatin regulation. Ubiquitination involves a sequential enzymatic cascade constituted by an activating enzyme (E1), a conjugating enzyme (E2), and a ligating enzyme (E3) that covalently bind Ub to the target protein.
Moreover, there are specific peptidases (deubiquitinases) that reverse the action of E3 ligases by removing the Ub from the proteins. Comparative genomics analysis suggests that the Ub system had a pre-eukaryotic origin [
68]. Ub, ER-associated Ub-conjugating enzymes, and genes potentially involved in ER-associated degradation have been identified in
E. histolytica [
69] and in
Acanthamoeba [
70].
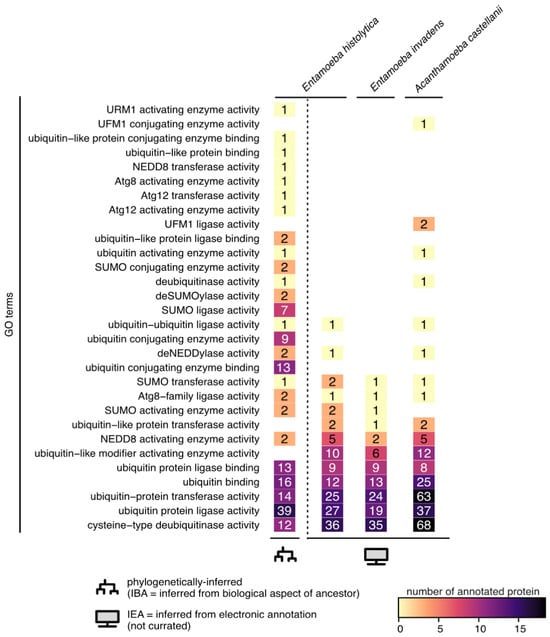
Figure 3 shows that many genes are currently annotated with functions related to protein ubiquitination in the reference genomes of
Entamoeba histolytica (
Entamoeba histolytica HM-1:IMSS) and
Acanthamoeba castellani (
Acanthamoeba castellani str. Neff), and in the genome of
Entamoeba invadens (
Entamoeba invadens IP1).
Figure 3. Ubiquitin-like associated function in pathogenic amoebae. Number of protein accessions annotated with ubiquitin-like function in the reference genomes of Entamoeba histolytica (Entamoeba histolytica HM-1:IMSS-taxon 294381), Entamoeba invadens (Entamoeba invadens IP1-taxon 370355), and Acanthamoeba castellani (Acanthamoeba castellani str. Neff-taxon 1257118). GO terms were retrieved from the QuickGo website (ebi.ac.uk/QuickGO) on the 9th of September 2023. E. histolytica HM-1:IMSS is the only taxon with phylogenetically inferred biological functions (IBA); all the others only have automatic computationally inferred annotations (IEA).
Other PTM signalling pathways known as UBL systems have been characterized by their similarity with the tertiary structure of Ub (a β-grasp fold). The UBL family includes neural precursor cell-expressed developmentally downregulated 8 (NEDD8), small ubiquitin-related modifier (SUMO), ubiquitin-fold modifier 1 (UFM1), ubiquitin-related modifier 1 (URM1), autophagy-related proteins 8 and 12 (ATG8 and ATG12), interferon-stimulated gene 15 (ISG15), and human leukocyte antigen-F adjacent transcript 10 (FAT10). Despite their structural similarities with the Ub system, UBL systems exhibit distinct enzymatic cascade structures with unique sets of enzymes. The understanding of the mechanistic aspects and biological roles of UBL pathways has significantly advanced in yeast, plant, and mammalian cells. A rich variety of UBLs have been found in parasitic protozoa [
71], with the exception of ISG15 and FAT10, which are involved in the immune and stress responses of multicellular organisms. All UBLs, with the exception of metazoan-specific UBLs and UFM1, are encoded by
Entamoeba species [
69].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11112670