Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
ST2 is a member of the interleukin (IL)-1 receptor family, whose gene is located on human chromosome 2q12. Alternative promoter splicing and 3′ processing of the mRNA are responsible for the production of two different forms: a soluble receptor, named sST2; or a transmembrane receptor, named ST2L. ST2 was first described in 1989.
- heart failure
- solubleST2
- natriuretic peptides
1. Introduction
Quantifying concentrations of circulating biomarkers plays a major role in most cardiovascular (CV) diseases, including heart failure (HF) [1].
An ideal biomarker in HF should be (1) measured non-invasively and at low cost, (2) highly sensitive to allow for the early detection of the disease, (3) unaffected or minimally affected by comorbid conditions, and (4) responsive to treatment effects [2]. The most established biomarkers in HF are B-type natriuretic peptide (BNP) and its co-secreted amino-terminal pro-peptide fragment (NT-proBNP), which reflect cardiac trans-mural wall stress. BNPs are strong predictors of HF presence and severity and provide prognostic information; therefore, BNP and NT-proBNP have a class 1 recommendation in the current European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) HF guidelines for these indications [3,4].
Beyond their well-established diagnostic role in acute and chronic setting, the role of BNP and NT-proBNP in risk stratification is gaining more momentum in clinical practice. In fact, low values of NPs at discharge reflect the achievement of greater decongestion, which correlate with a lower risk of re-hospitalization and death. In addition, the pre-discharge value can be used to determine the intensity of monitoring and the timing for follow-up visits [5].
However, there are important limitations to natriuretic peptide (NP) testing in HF. Most important is the impact caused by conditions commonly associated with HF such as atrial fibrillation (AF), kidney dysfunction and obesity, as well as a wide range of cardiac and non-cardiac abnormalities associated with an increase in parietal tension without necessarily being linked to fluid retention [5]. NP concentrations also vary substantially with age and sex, which introduces difficulties in using thresholds for decision making. Beyond these issues, the concentrations of BNP and NT-proBNP only reflect one aspect of the considerably complex pathophysiology of HF. Accordingly, a broader palette of biomarkers would be expected to provide an important depth of understanding of individuals affected by the diagnosis.
Numerous other biomarkers have been evaluated in HF and are under investigation. Some of these have been more convincing and are used in some clinics today. Of particular prominence is soluble ST2 (sST2) [1], which was first classified as an indicator of ventricular myocyte stress [6], but is mainly produced in extracardiac tissues [7] in response to inflammatory and fibrotic stimuli [8], representing an indicator of the myocardial fibrotic process and a predictor of cardiac remodeling [9,10,11].
2. sST2 Biology
ST2 is a member of the interleukin (IL)-1 receptor family [12], whose gene is located on human chromosome 2q12. Alternative promoter splicing and 3′ processing of the mRNA are responsible for the production of two different forms: a soluble receptor, named sST2; or a transmembrane receptor, named ST2L [2,8,13]. ST2 was first described in 1989 [14,15].
The literature mistakenly called ST2 a “suppressor of tumorigenicity 2”, when, in fact, the original name it was given was “growth stimulation expressed gene 2”, then renamed “serum stimulation-2”, as it was first discovered to function as a mediator of type 2 inflammatory responses [16].
Its role as a cardiac marker was suggested in 2002 by Weinberg et al. [17], analyzing the expression of 7.000 genes in cardiomyocytes undergoing mechanical strain and noting that myocardial transcripts of ST2 increased significantly in response to this stimulus. This is curious and important, as the main source of sST2 in the circulation in patients with HF does not appear to be the heart. Indeed, it has been shown that type 2 pneumocytes represent a relevant source of sST2 in HF patients and concentrations of sST2 in pulmonary edema from individuals with HF are strongly correlated to blood values [7]. This link to pulmonary pathophysiology may explain why sST2 correlates with the presence and severity of pulmonary congestion in HF [18]. This is in contrast to NPs, which are also upregulated in HF and correlate with pulmonary congestion, but are only expressed in cardiomyocytes and not the lungs. For this reason, an additional role of sST2 relative to NPs for the evaluation of the HF phenotype and prognosis seems likely from a biological perspective.
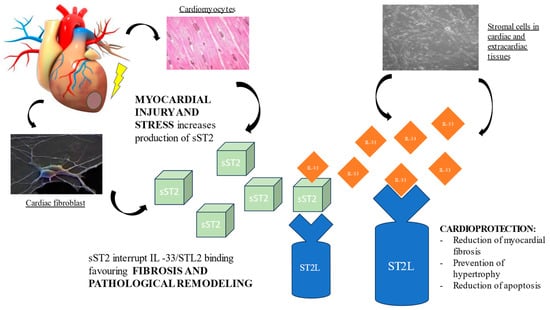
The cognate ligand of ST2 is interleukin-33 (IL-33), a cardiac fibroblast protein released by stromal cells in cardiac and extracardiac tissues. Depending on co-stimulatory factors, IL-33 can act either as a pro- or anti-inflammatory cytokine. At the cardiac level, the ST2L/IL-33 interaction initiates a complex cardioprotective biochemical cascade, which prevents cardiomyocyte hypertrophy, apoptosis, and myocardial fibrosis, thereby improving cardiac function. However, when the heart is subjected to damage or mechanical stress, cardiomyocytes and cardiac fibroblasts secrete sST2, which, competing with ST2L for the IL-33 binding site, antagonizes the cardioprotective effect, contributing to myocardial fibrosis and ventricular remodeling [12,19,20]. (Figure 1) Hence, the activation of the ST2L/IL-33 pathway is a beneficial adaptive response in cardiac disease, which is offset by sST2 secretion.

Figure 1. Pathological role of sST2 in promoting fibrosis and ventricular remodeling.
The “inflammatory hypothesis” of atherosclerosis implies that the presence of inflammation favors the formation, growth, and, finally, the instability of atherosclerotic plaques, favoring the onset of cardiovascular events [21]. The IL-33-ST2L pathway could inhibit the development of atherosclerosis through the immune response toward a T helper 2, macrophage 2 phenotype, while high sST2 values could promote plaque development, sequestering IL-33 [22]. As a result, in patients with non-ST-elevation acute coronary syndrome, the level of serum sST2 might be a useful predictive marker of plaque vulnerability [23]. From a neurological point of view, sST2 levels increase in patients with mild cognitive impairment, suggesting that impaired IL-33/ST2 signaling may contribute to the pathogenesis of Alzheimer’s disease [24] and an elevation in sST2 serum concentration represents and tracks disease progression.
sST2 also appears to be involved in the pathogenesis of cancers, trying to counterbalance the tumorigenesis effect of IL-33/ST2, and could therefore be used for non-invasive diagnostic tests, as a prognostic marker and for treatment monitoring [25]. For example, in gastric cancer, sST2 was significantly associated with a more advanced tumor stage (p = 0.018), metastatic disease (p = 0.014), and was significantly correlated with the duration of the disease (p = 0.0017) [26]. Similarly, serum levels of IL-33 and sST2 were significantly higher in breast cancer patients in comparison with healthy volunteers [25,27].
ST2L is a cell-surface marker of T helper type 2 (Th2) lymphocytes and, therefore, IL-33/ST2 has an essential role in immune regulation. As a result, it has been associated with diseases characterized by a predominantly Th2 response, such as asthma, pulmonary fibrosis, rheumatoid arthritis, collagen vascular diseases, sepsis, trauma, fibroproliferative diseases and ulcerative colitis [25]. IL-33/ST2 also has a profibrotic role in the pathogenesis of hepatic diseases. In this regard, sST2 has an opposite function, and its elevation in liver cirrhosis, hepatocellular carcinoma and hepatitis B infection could be a sign of a positive regulatory loop in the remission of these diseases [28,29].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10110468
This entry is offline, you can click here to edit this entry!
