Mesenchymal stem cells (MSCs) have a high tropism for the hypoxic microenvironment of tumors. The combination of nanoparticles in MSCs decreases tumor growth in vitro as well as in rodent models of cancers in vivo. Covalent conjugation of nanoparticles with the surface of MSCs can significantly increase the drug load delivery in tumor sites. Nanoparticle-based anti-angiogenic systems (gold, silica and silicates, diamond, silver, and copper) prevented tumor growth in vitro. For example, glycolic acid polyconjugates enhance nanoparticle drug delivery and have been reported in human MSCs. Labeling with fluorescent particles (coumarin-6 dye) identified tumor cells using fluorescence emission in tissues; the conjugation of different types of nanoparticles in MSCs ensured success and feasibility by tracking the migration and its intratumor detection using non-invasive imaging techniques.
- nanoparticles
- mesenchymal stem cells (MSC)
- homing
- tumors
1. Mesenchymal Stem Cells as Drug Vehicles: Nanoparticles as Drug Carriers
Does Transplanted Mesenchymal Stem Cells (MSC) Exert Antitumoral or Favor Tumorigenesis?
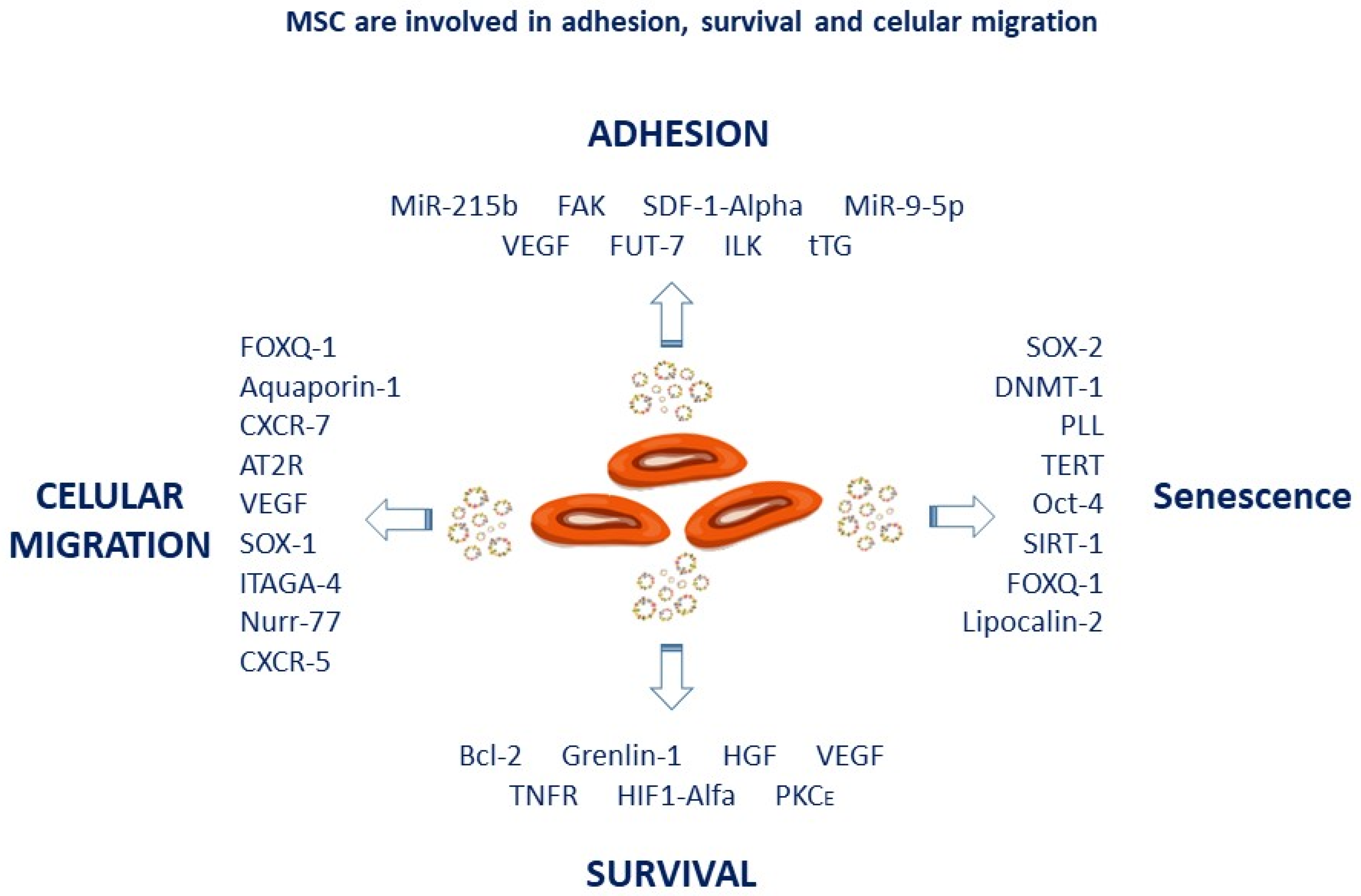
2. Mesenchymal Stem Cell as Vehicles for Drug Delivery against Tumoral Cells
2.1. Interaction between Nanoparticles (NP) and Stem Cells
| Receptor-ligand binding | PTX Gencitabine Gadolinium Nanoparticles (load with cumarines) |
Leukemia Pancreas cancer Diagnosis of cancer Glioma |
| Transduction | mR-133 | Miocardic infarct (acute) |
| Transfection | TRAILs | Lung metastases |
| HIF-1 Alpha | Miocardic infarct (acute) |
This entry is adapted from the peer-reviewed paper 10.3390/mi14112068
References
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663.
- Wang, P.; Zhang, J.; Zhang, Q.; Liu, F. Mesenchymal stem cells loaded with Ad5-Ki67/IL-15 enhance oncolytic adenovirotherapy in experimental glioblastoma. Biomed. Pharmacother. 2023, 157, 114035.
- Kiran, S.; Dwivedi, P.; Kumar, V.; Price, R.L.; Singh, U.P. Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss. Cells 2021, 10, 2016.
- Park, J.S.; Suryaprakash, S.; Lao, Y.H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16.
- Naderi-Meshkin, H.; Bahrami, A.R.; Bidkhori, H.R.; Mirahmadi, M.; Ahmadiankia, N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol. Int. 2015, 39, 23–34.
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768.
- Gao, W.; Zhang, L. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015, 23, 619–626.
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610.
- Liang, J.; Huang, W.; Yu, X.; Ashraf, A.; Wary, K.K.; Xu, M.; Millard, R.W.; Ashraf, M.; Wang, Y. Suicide gene reveals the myocardial neovascularization role of mesenchymal stem cells overexpressing CXCR4 (MSC(CXCR4)). PLoS ONE 2012, 7, e46158.
- Timin, A.S.; Peltek, O.O.; Zyuzin, M.V.; Muslimov, A.R.; Karpov, T.E.; Epifanovskaya, O.S.; Shakirova, A.I.; Zhukov, M.V.; Tarakanchikova, Y.V.; Lepik, K.V.; et al. Safe and Effective Delivery of Antitumor Drug Using Mesenchymal Stem Cells Impregnated with Submicron Carriers. ACS Appl. Mater Interfaces 2019, 11, 13091–13104.
- Xia, J.; Tsai, A.C.; Cheng, W.; Yuan, X.; Ma, T.; Guan, J. Development of a microdevice-based human mesenchymal stem cell-mediated drug delivery system. Biomater. Sci. 2019, 7, 2348–2357.
- Thanuja, M.Y.; Anupama, C.; Ranganath, S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Adv. Drug Deliv. Rev. 2018, 132, 57–80.
- Zhang, R.; Zhang, Q.; Zou, Z.; Li, Z.; Jin, M.; An, J.; Li, H.; Ma, J. Curcumin Supplementation Enhances Bone Marrow Mesenchymal Stem Cells to Promote the Anabolism of Articular Chondrocytes and Cartilage Repair. Cell Transplant. 2021, 30, 963689721993776.
- Kim, G.B.; Shon, O.J.; Seo, M.S.; Choi, Y.; Park, W.T.; Lee, G.W. Mesenchymal Stem Cell-Derived Exosomes and Their Therapeutic Potential for Osteoarthritis. Biology 2021, 10, 285.
- Gothelf, Y.; Abramov, N.; Harel, A.; Offen, D. Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin. Transl. Med. 2014, 3, 21.
- Kronsteiner, B.; Peterbauer-Scherb, A.; Grillari-Voglauer, R.; Redl, H.; Gabriel, C.; van Griensven, M.; Wolbank, S. Human mesenchymal stem cells and renal tubular epithelial cells differentially influence monocyte-derived dendritic cell differentiation and maturation. Cell. Immunol. 2011, 267, 30–38.
- Ryan, A.; Barry, F. Mesenchymal stem/stromal cell therapy: Mechanism of action and host response. In The Biology of Therapeutic Applications of Mesenchimal Cells Chapter 9, 1st ed.; Atkinson, K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 29, pp. 426–440.
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49.
- Selleri, S.; Bifsha, P.; Civini, S.; Pacelli, C.; Dieng, M.M.; Lemieux, W.; Jin, P.; Bazin, R.; Patey, N.; Marincola, F.M.; et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 2016, 7, 30193–30210.
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143.
- Bassi, E.J.; Aita, C.A.; Câmara, N.O. Immune regulatory properties of multipotent mesenchymal stromal cells: Where do we stand? World J. Stem Cells 2011, 3, 1–8.
- Omoto, M.; Katikireddy, K.R.; Rezazadeh, A.; Dohlman, T.H.; Chauhan, S.K. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6631–6638.
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F., 3rd. Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells 2011, 29, 11–19.
- Torsvik, A.; Røsland, G.V.; Svendsen, A.; Molven, A.; Immervoll, H.; McCormack, E.; Lønning, P.E.; Primon, M.; Sobala, E.; Tonn, J.C.; et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: Putting the research field on track–letter. Cancer Res. 2010, 70, 6393–6396.
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004, 96, 1593–1603.
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.C.; Tanager, K.S.; Yoon, E.; Kidwell; et al. Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017, 18, 1215–1228.
- Huang, J.C.; Basu, S.K.; Zhao, X.; Chien, S.; Fang, M.; Oehler, V.G.; Appelbaum, F.R.; Becker, P.S. Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J. 2015, 5, e302.
- Rhee, K.J.; Lee, J.I.; Eom, Y.W. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033.
- Ohlsson, L.B.; Varas, L.; Kjellman, C.; Edvardsen, K.; Lindvall, M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 2003, 75, 248–255.
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946.
- Clarke, M.R.; Imhoff, F.M.; Baird, S.K. Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol. Carcinog. 2015, 54, 1214–1219.
- Zhu, Y.; Sun, Z.; Han, Q.; Liao, L.; Wang, J.; Bian, C.; Li, J.; Yan, X.; Liu, Y.; Shao, C.; et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009, 23, 925–933.
- Ramasamy, R.; Lam, E.W.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007, 21, 304–310.
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247.
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827.
- Lee, H.Y.; Hong, I.S. Targeting Liver Cancer Stem Cells: An Alternative Therapeutic Approach for Liver Cancer. Cancers 2020, 12, 2746.
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559.
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.M.; Sonoyama, W.; Zheng, J.J.; Baker, C.C. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 2006, 24, 1095–1103.
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to Optimize Adult Stem Cell Therapy for Tissue Regeneration. Int. J. Mol. Sci. 2016, 17, 982.
- Ren, G.; Su, J.; Zhang, L.; Zhao, X.; Ling, W.; L’huillie, A.; Zhang, J.; Lu, Y.; Roberts, A.I.; Ji, W.; et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009, 27, 1954–1962.
- Pan, Q.; Fouraschen, S.M.; de Ruiter, P.E.; Dinjens, W.N.; Kwekkeboom, J.; Tilanus, H.W.; van der Laan, L.J. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp. Biol. Med. 2014, 239, 105–115.
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402.
- Stultz, B.G.; McGinnis, K.; Thompson, E.E.; Lo Surdo, J.L.; Bauer, S.R.; Hursh, D.A. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy 2016, 18, 336–343.
- Conforti, A.; Starc, N.; Biagini, S.; Tomao, L.; Pitisci, A.; Algeri, M.; Sirleto, P.; Novelli, A.; Grisendi, G.; Candini, O.; et al. Resistance to neoplastic transformation of ex-vivo expanded human mesenchymal stromal cells after exposure to supramaximal physical and chemical stress. Oncotarget 2016, 7, 77416–77429.
- Faraday, M.X. The bakerian lecture—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181.
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, W.M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150.
- Ramos, M.A.D.S.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179.
- Melo, M.A., Jr.; Santos, L.S.S.; Gonçalves, M.D.C.; Nogueira, A.F. Preparation of silver and gold nanoparticles: A simple method to introduce nanotechnology into teaching laboratories. Quim. Nova 2012, 35, 1872–1878.
- Iravani, S. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; Chapter 2; pp. 15–31.
- Kumar, M.; Varshney, L.; Francis, S. Radiolytic formation of ag clusters in aqueous polyvinyl alcohol solution and hydrogel matrix. Radiat. Phys. Chem. 2005, 73, 21–27.
- Mody, V.V.; Nounou, M.I.; Bikram, M. Novel nanomedicine-based MRI contrast agents for gynecological malignancies. Adv. Drug Deliv. Rev. 2009, 61, 795–807.
- Abdal Dayem, A.; Lee, S.B.; Cho, S.G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761.
- Shin, T.H.; Lee, D.Y.; Ketebo, A.A.; Lee, S.; Manavalan, B.; Basith, S.; Ahn, C.; Kang, S.H.; Park, S.; Lee, G. Silica-Coated Magnetic Nanoparticles Decrease Human Bone Marrow-Derived Mesenchymal Stem Cell Migratory Activity by Reducing Membrane Fluidity and Impairing Focal Adhesion. Nanomaterials 2019, 9, 1475.
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434.
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for engineering stem cell responses. Adv. Healthc. Mater. 2015, 4, 1600–1627.
- Li, J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007.
- Ko, W.-K.; Heo, D.N.; Moon, H.-J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.-C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid Interface Sci. 2015, 438, 68–76.
- Vácha, R.; Martinez-Veracoechea, F.J.; Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011, 11, 5391–5395.
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448.
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailänder, V. How shape influences uptake: Interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 2012, 8, 2222–2230.
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671.
- Lorenz, S.; Hauser, C.P.; Autenrieth, B.; Weiss, C.K.; Landfester, K.; Mailänder, V. The softer and more hydrophobic the better: Influence of the side chain of polymethacrylate nanoparticles for cellular uptake. Macromol. Biosci. 2010, 10, 1034–1042.
- Zhang, R.; Lee, P.; Lui, V.C.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.; Wong, K.K. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1949–1959.
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Štangar, U.L.; Bergant, M.; Mahne, D. Photocatalytic and antibacterial activity of TiO2 and au/TiO2 nanosystems. Nanotechnology 2007, 18, 375709.
- Jiang, M.; Wu, B.; Sun, Y.; Ding, Y.; Xie, Y.; Liu, L.; Cao, Y. Toxicity of ZnO nanoparticles (NPs) to THP-1 macrophages: Interactions with saturated or unsaturated free fatty acids. Toxicol. Mech. Methods 2019, 29, 291–299.
- Fan, L.; Wei, A.; Gao, Z.; Mu, X. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomed. Pharmacother. 2023, 161, 114451.
- Wu, J.; Wu, K.; Lin, F.; Luo, Q.; Yang, L.; Shi, Y.; Song, G.; Sung, K.L. Mechano-growth factor induces migration of rat mesenchymal stem cells by altering its mechanical properties and activating ERK pathway. Biochem. Biophys. Res. Commun. 2013, 441, 202–207.
- Auffinger, B.; Morshed, R.; Tobias, A.; Cheng, Y.; Ahmed, A.U.; Lesniak, M.S. Drug-loaded nanoparticle systems and adult stem cells: A potential marriage for the treatment of malignant glioma? Oncotarget 2013, 4, 378–396.
- Borkowska, M.; Siek, M.; Kolygina, D.V.; Sobolev, Y.I.; Lach, S.; Kumar, S.; Cho, Y.K.; Kandere-Grzybowska, K.; Grzybowski, B.A. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 2020, 15, 331–341.
- Shrestha, S.; Jiang, P.; Sousa, M.H.; Morais, P.C.; Mao, Z.; Gao, C. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 245–256.
- Schäfer, R.; Kehlbach, R.; Müller, M.; Bantleon, R.; Kluba, T.; Ayturan, M.; Siegel, G.; Wolburg, H.; Northoff, H.; Dietz, K.; et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy 2009, 11, 68–78.
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942.
- Cao, Y.; Zhang, S.; Ma, M.; Zhang, Y. Fluorinated PEG-PEI Coated Magnetic Nanoparticles for siRNA Delivery and CXCR4 Knockdown. Nanomaterials 2022, 12, 1692.
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944.
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016.
- Rofe, A.P.; Pryor, P.R. Purification of Lysosomes Using Supraparamagnetic Iron Oxide Nanoparticles (SPIONs). Cold Spring Harb. Protoc. 2016, 4, pdb.prot084822.
- LaConte, L.; Nitin, N.; Bao, G. Magnetic nanoparticle probes. Mater. Today 2005, 8, 32–38.
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641.
- Norizadeh-Abbariki, T.; Mashinchian, O.; Shokrgozar, M.A.; Haghighipour, N.; Sen, T.; Mahmoudi, M. Superparamagnetic nanoparticles direct differentiation of embryonic stem cells into skeletal muscle cells. J. Biomater. Tissue Eng. 2014, 4, 579–585.
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358.
- Chen, Y.C.; Hsiao, J.K.; Liu, H.M.; Lai, I.Y.; Yao, M.; Hsu, S.C.; Ko, B.-S.; Chen, Y.C.; Yang, C.S.; Huang, D.M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2010, 245, 272–279.
- Kostura, L.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F.; Bulte, J.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004, 17, 513–517.
- Sun, J.; Liu, X.; Huang, J.; Song, L.; Chen, Z.; Liu, H.; Li, Y.; Zhang, Y.; Gu, N. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci. Rep. 2014, 4, 5125.
- Wang, Q.; Chen, B.; Ma, F.; Lin, S.; Cao, M.; Li, Y.; Gu, N. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding rna inzeb2. Nano Res. 2017, 10, 626–642.
- Liu, Y.; Huang, W.; Wang, H.; Lu, W.; Guo, J.; Yu, L.; Wang, L. Influence of SPIO labelling on the function of BMSCs in chemokine receptors expression and chemotaxis. PeerJ 2023, 11, e15388.
- Han, J.; Kim, B.; Shin, J.Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J.S.; Lee, Y.; Lee, N.; Hyeon, T. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819.
- Rafieepour, A.; Azari, M.R.; Khodagholi, F.; Jaktaji, J.P.; Mehrabi, Y.; Peirovi, H. The effect of single and combined exposures to magnetite and polymorphous silicon dioxide nanoparticles on the human A549 cell line: In vitro study. Environ. Sci. Pollut. Res. Int. 2019, 26, 31752–31762.
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321.
- Mirshafiee, V.; Kim, R.; Mahmoudi, M.; Kraft, M.L. The importance of selecting a proper biological milieu for protein corona analysis in vitro: Human plasma versus human serum. Int. J. Biochem. Cell Biol. 2016, 75, 188–195.
- Tekin, H.; Sanchez, J.G.; Landeros, C.; Dubbin, K.; Langer, R.; Khademhosseini, A. Controlling spatial organization of multiple cell types in defined 3D geometries. Adv. Mater. 2012, 24, 5543–5547.
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated biomaterials for engineering 3D tissues. Adv. Mater. 2012, 24, 1782–1804.
- Van, V.T.; Ponsaerts, P.; Berneman, Z.N. Mrna-based gene transfer as a tool for gene and cell therapy. Curr. Opin. Mol. Ther. 2007, 9, 423–431.
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 3619.
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691.
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519.
- Heo, S.J.; Szczesny, S.E.; Kim, D.H.; Saleh, K.S.; Mauck, R.L. Expansion of mesenchymal stem cells on electrospun scaffolds maintains stemness, mechano-responsivity, and differentiation potential. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 808–815.
- Jan, N.; Madni, A.; Khan, S.; Shah, H.; Akram, F.; Khan, A.; Ertas, D.; Bostanudin, M.F.; Contag, C.H.; Ashammakhi, N.; et al. Biomimetic cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for biomedical applications. Bioeng. Transl. Med. 2022, 8, e10441.
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J Nanobiotechnol. 2022, 20, 542.
- Aviram, I.; Shaklai, N. The association of human erythrocyte catalase with the cell membrane. Arch. Biochem. Biophys. 1981, 212, 329–337.
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Red Blood Cell-Membrane-Coated Poly(Lactic-co-glycolic Acid) Nanoparticles for Enhanced Chemo- and Hypoxia-Activated Therapy. ACS Appl. Bio Mater. 2019, 2, 4077–4086.
- Suryaprakash, S.; Lao, Y.H.; Cho, H.Y.; Li, M.; Ji, H.Y.; Shao, D.; Hu, H.; Quek, C.H.; Huang, D.; Mintz, R.L.; et al. Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019, 19, 1701–1705.
- Su, Y.; Zhang, T.; Huang, T.; Gao, J. Current advances and challenges of mesenchymal stem cells-based drug delivery system and their improvements. Int. J. Pharm. 2021, 600, 120477.
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131.
- Yang, Y.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9, 120–122.
- Su, J.; Chen, X.; Huang, Y.; Li, W.; Li, J.; Cao, K.; Cao, G.; Zhang, L.; Li, F.; Roberts, A.I.; et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014, 21, 388–396.
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021, 9, 1088–1103.
- Pereboeva, L.; Komarova, S.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells 2003, 21, 389–404.
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392.
- Dhada, K.S.; Hernandez, D.S.; Suggs, L.J. In Vivo Photoacoustic Tracking of Mesenchymal Stem Cell Viability. ACS Nano 2019, 13, 7791–7799.
- Wang, W.; Deng, Z.; Xu, X.; Li, Z.; Jung, F.; Ma, N.; Lendlein, A. Functional Nanoparticles and their Interactions with Mesenchymal Stem Cells. Curr. Pharm. Des. 2017, 23, 3814–3832.
- Xie, X.; Zhang, Q.; Zhou, T.; Ma, Q.; Liao, J. The Review of Nanomaterials Inducing the Differentiation of Stem Cells into Chondrocyte Phenotypes in Cartilage Tissue Engineering. Curr. Stem Cell Res. Ther. 2018, 13, 600–607.
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63.
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483.
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 26, 12386–12397.
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499.
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337.
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445.
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692.
