Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Mitophagy is crucial for maintaining mitochondrial quality. The endosomal–lysosomal system is a more accessible pathway through which subtypes of extracellular vesicles, which also contain mitochondrial constituents, are released for disposal. The inclusion of mitochondrial components into extracellular vesicles occurs in the setting of mild mitochondrial damage and during impairment of lysosomal function. By releasing mitochondrial-derived vesicles, cells limit the unload of mitochondrial damage-associated molecular patterns with proinflammatory activity.
- damage-associated molecular patterns (DAMPs)
- endosomal–lysosomal system
- exosomes
- extracellular vesicles
1. Introduction
Mitophagy is a major mechanism of mitochondrial quality control. Mitophagy involves the degradation and recycling of damaged or inefficient mitochondria to maintain a healthy pool of organelles and ensure adequate energy provision to cells [1]. However, its assessment in vivo is challenging, which hampers its translational applications [2].
The endosomal–lysosomal system encompasses organelles and membranous components that form the endocytic pathway. Through the endosomal–lysosomal system, various cargo molecules are internalized and recycled. Furthermore, this pathway has emerged as a relevant and more accessible component of the mitochondrial quality control system [3][4]. Exosomes, a subtype of extracellular vesicles (EVs) with a diameter of 50–150 nm, are generated and released by the endosomal–lysosomal system. Exosomes are produced from intraluminal vesicles (ILVs), which originate from early endosomes undergoing inward budding of discrete membrane domains that subsequently evolve into multivesicular bodies (MVBs) [5][6][7][8]. MVBs are usually addressed to lysosomes for cargo degradation and recycling. However, under specific stimulation, MVBs can be shuttled toward the plasma membrane for exocytic fusion and subsequent extracellular release of ILVs [5][6]. Under these circumstances, ILVs are called exosomes.
Growing evidence has shown that, depending on the severity of mitochondrial damage, cells can selectively target mitochondrial components for lysosomal degradation and regulate the packaging of mitochondrial constituents into EVs [9]. Mitochondrial disposal via EVs seems to be an alternative strategy to canonical organelle degradation and recycling by mitophagy [9]. The EV pathway of mitochondrial quality control is triggered in the setting of mild mitochondrial damage to avoid wholesale organelle disposal while preventing the release of unpackaged damaged mitochondrial components. The latter would otherwise act as proinflammatory damage-associated molecular patterns (DAMPs) and install an inflammatory milieu [9]. Mitochondrial clearance has also been shown to be accomplished via the endosomal pathway and large EV generation when lysosomal degradation is inhibited [10].
The generation of mitochondrial-derived vesicles (MDVs), small vesicles that shuttle mitochondrial constituents to other organelles, is accomplished through the sorting of mitochondrial components via two different pathways. The first involves the delivery of mitochondrial material to EVs through sorting nexin 9 (SNX9)-dependent MDVs [11]. This vesicle class has been shown to participate in mitochondrial antigen presentation [11]. The second pathway targets MDVs containing damaged mitochondrial components to lysosomes for degradation in a process that is regulated by the mitophagy mediator Parkin [11]. Therefore, the characterization of MDV subtypes represents a unique tool for investigating the dynamics of mitochondrial quality control in health and disease [12]. MDVs also enable intercellular communication with either beneficial or harmful effects on recipient cells, depending on the cellular source as well as the nature of the cargo and the originating stimulus [13]. To further complicate the matter, a distinct population of EVs containing mitochondrial material—mitovesicles—has recently been described and attributed specific signaling functions [14]. All these factors contribute to a limited exploitation of MDVs in the clinical setting, for which a deeper characterization of MDV biology is needed [15].
2. Mitochondrial-Derived Vesicles
First identified in 2008 by Neuspiel et al. [16], MDVs are single- or double-membrane vesicles, generated, respectively, from the outer mitochondrial membrane (OMM) or inner mitochondrial membrane (IMM) and portions of the mitochondrial matrix. Three independent criteria are used for specifically defining MDVs: (i) generation independent of dynamin-related protein 1 (DRP1); (ii) small size (diameter of 70–150 nm); and (iii) selectivity of the cargo [16][17].
Mitochondria have inherited, from their bacterial ancestors, the ability to load their contents into vesicles for long-distance delivery as a constitutive and evolutionarily conserved process [18][19]. These similar prokaryotic vesicles are generated by local evaginations of mitochondrial membranes, vesicle budding, and release into the extracellular compartment. Bacteria release two main types of EVs: outer membrane vesicles (OMVs), generated from the outer plasma membrane layer, and outer–inner membrane vesicles (O–IMVs), containing both outer and inner membrane layer components [20]. OMVs mainly include bacterial lipopolysaccharides (LPSs) from the outer layer, peptidoglycans of the periplasmic space, and unstructured and organelle-free cytosol [21][22]. Conversely, ATP molecules, DNA, and proteins from the cytoplasm and the inner membrane are mostly included in O–IMVs [21][22]. EVs are implicated in multiple bacterial activities, from intracolonial signaling via quorum sensing [23] to intercellular communication via proteins shuttling, as well as in the modulation of immunogenic host invasion, elimination of bacterial competitors, and formation of biofilms [24].
MDVs in eukaryotic cells have more specialized roles compared with their prokaryotic counterparts and have evolved for different purposes [18]. In the setting of early mitochondrial stress, MDVs can shuttle oxidized cargo to lysosomes for degradation, or unload it in the extracellular compartment via EVs, thereby regulating mitochondrial mass more rapidly than mitophagy [3][4][9]. MDVs are highly heterogeneous, and several subtypes have been identified, which also depend on the cell type [25][26][27]. Their heterogeneity is reflected by the multiple processes in which they are involved, including mitochondrial fission, biogenesis of peroxisomes, resistance to oxidative stress and infections, and innate immunity signaling [11][28][29][30][31]. Alterations in the mechanisms generating MDVs have been associated with several pathological conditions, such as neurodegeneration and cardiomyocyte damage under ischemia/hypoxia [4][32][33]. Moreover, altered MDV generation and release have been associated with aging, autoimmune diseases, cancer, and infections [11][34][35].
MDV generation is a housekeeping process that occurs at a basal level under physiological conditions [25][36] and is enhanced during pathological stress. An increase in the production of reactive oxygen species (ROS) inflicts damage on proteins, lipids, and nucleic acids, and cells can trigger MDV formation to allow clearance of abnormal mitochondrial particles [28][36][37]. In the setting of mild oxidative stress, the oxidation of mitochondrial membrane proteins initiates the local activation of phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1)/Parkin, guiding the budding of oxidized membrane proteins and their inclusion into vesicles [4][11].
Under physiological conditions, PINK1 is constitutively imported through the mitochondrial import channel of the outer membrane and, when inside the organelle, is cleaved by the protease of the inner membrane (PARL) [38]. Upon cleavage, PINK1 is retro-translocated to the cytoplasm for rapid proteasomal degradation [39], while Parkin resides in the cytosol in the form of an autoinhibited E3 ubiquitin-ligase [33]. Under mild stress, mitochondrial depolarization disrupts the activity of mitochondrial import channels and blocks the internalization of PINK1 that becomes stalled at the import channel or at the outer mitochondrial membrane [40][41]. Here, PINK1 undergoes autophosphorylation and, in turn, phosphorylates the ubiquitin protein and the ubiquitin-like domains of Parkin. These events stabilize Parkin in an active state and facilitate MDV generation and release [40][41].
Matheoud et al. [11] have shown that the biogenesis of MDVs also requires recruitment of the Ras-related protein (Rab9) and SNX9, although the regulation of this process remains unclear. Rab9 is a small GTPase located at the trans-Golgi network and late endosomes, and is implicated in pathways regulating the endo−lysosomal trafficking [42]. Conversely, SNX9 binds directly to the regulator of endocytosis, mediated by the protein clathrin [43]. This pathway generates MDVs for mediating mitochondrial antigen presentation after proteasomal breaking of mitochondrial components into lysosomes and loading of mitochondrial antigens onto major histocompatibility complex class I (MHC I) molecules at the endoplasmic reticulum for their subsequent transfer to cell surface [11][44][45] (Figure 1). Through this pathway, MDVs can regulate survival, development, activation, and differentiation of immune cells (e.g., T-lymphocytes and macrophages) [44][46][47][48]. The destination of MDVs to MVBs for final lysosomal fusion and degradation has also been described as a mechanism to selectively package and dispose mitochondrial constituents via EVs [28][49]. This process prevents the release of oxidized mitochondrial DAMPs with proinflammatory properties [28][49].
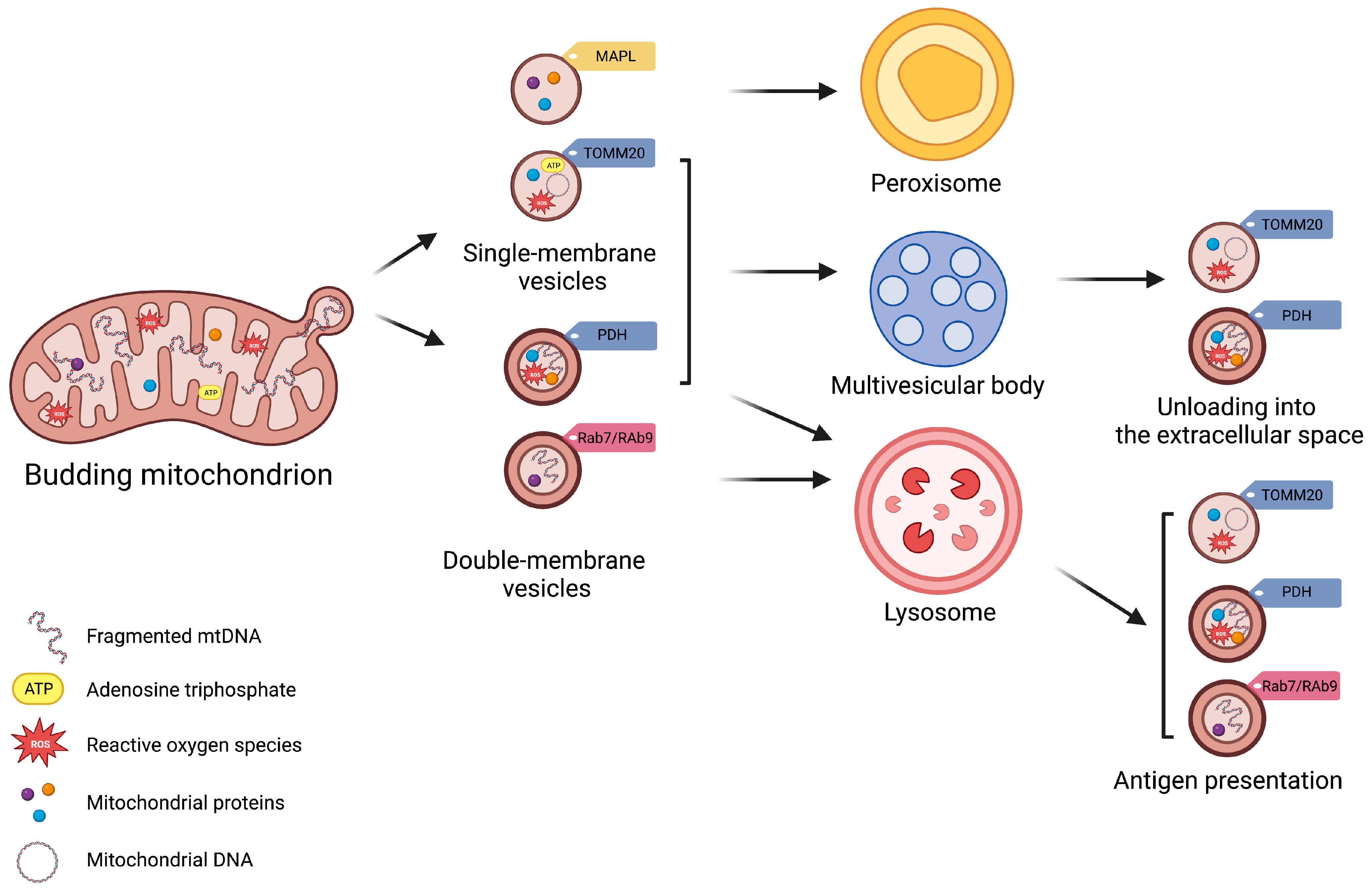
Figure 1. Schematic Representation of Subtypes of Mitochondrial-Derived Vesicles. Mitochondrial-derived vesicles (MDVs) can be classified according to their membrane composition and cargo selection. Based on membrane structure and composition, MDVs can be distinguished as single- or double-membrane vesicles. Single-membrane MDVs incorporate outer mitochondrial membrane proteins, while double-membrane MDVs include outer and inner mitochondrial membrane proteins and constituents of the mitochondrial matrix. According to cargo and membrane protein markers, MDV subtypes include single-membrane MDVs that bear mitochondrial-anchored protein ligase (MAPL) and the import channel translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20), and double-membrane MDVs with pyruvate dehydrogenase (PDH). Vesicle subtypes follow different degradative pathways. Peroxisome is the final destination of single-membrane MDVs with MAPL, while those with TOMM are excreted via multivesicular bodies (MVBs) as exosomes. Double-membrane MDVs with PDH are also released via MVBs. Finally, Ras-related protein (Rab) 7-/9-guided MDV generation mediate mitochondrial antigen presentation via major histocompatibility complex (MHC) class I.
Vesicle generation is guided by specific mitochondrial compartments. In particular, β-barrel proteins [50], the mitochondrial-anchored protein ligase (MAPL or Mul1) [51], and the import channel translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20) assist in the formation of single-membrane MDVs [52]. Double-membrane MDVs, instead, are formed by outer and inner mitochondrial membranes that also encapsulate matrix proteins [3][4][16][25][28][53]. These vesicles incorporate specific oxidative phosphorylation (OXPHOS) complexes (e.g., III and IV) of the inner mitochondrial membrane and iron–sulfur clusters to avoid mitochondrial iron overload and remove irreversibly damaged proteins [19]. MDVs may also encapsulate mitochondrial enzymes, including pyruvate dehydrogenase (PDH) and those of the mitochondrial matrix involved in the tricarboxylic acid (TCA) cycle, fatty acids β-oxidation [19], and the antioxidant enzyme superoxide dismutase 2 (SOD2) [28][30]. Among other signaling molecules, mitochondrial DNA (mtDNA) can also be engulfed in MDVs, which has been related to systemic inflammation in several disease conditions [12].
3. Mitovesicles
Recently, D’Acunzo et al. [54] published a validated experimental approach to purify and separate different EV subpopulations in vivo from the brain extracellular matrix. These EVs have distinct morphology as well as protein and lipid composition compared with microvesicles and exosomes [54]. The authors coined the term “mitovesicles” to refer to these EVs that bear mitochondrial components including proteins, lipids, and mtDNA [14]. D’Acunzo et al. [54] have established that mitovesicles are distinct from intracellular mitochondria, being 10-fold smaller and having a narrower intermembrane space of about 6 nm, compared with 20 nm in native organelles [55].
Mitovesicles also lack several mitochondrial structures, such as cristae, mitochondrial ribosomes, and proteins constitutively found in mitochondria, including the import channel TOMM20 [56]. Interestingly, these vesicles share some features with MDVs described earlier that bud from the mitochondrial surface, including the outer mitochondrial membrane, the inner mitochondrial membrane, and mitochondrial matrix, and are targeted to MVBs [3]. Thus, the possibility that MDVs can be released into the extracellular space as mitovesicles after fusion of MVBs with the plasma membrane cannot be ruled out [57]. However, the mechanisms responsible for mitovesicle generation from mitochondria and their secretion into the extracellular space remain unclear. Moreover, MDVs are heterogeneous, and some of the characteristics of these vesicles are not shared with mitovesicles. Indeed, MAPL, TOMM20, and vacuolar protein sorting-associated protein 35 (VPS35) [3] have not been found in mitovesicles [14]. Furthermore, the tetraspanin CD63, a component of membranes in late endosomes and lysosomes (also indicated as lysosomal associated membrane protein-3 (LAMP-3)), has not been found in mitovesicles, but only in exosomes and MDVs [58][59]. This suggests that distinct mechanisms might be in place to generate different subsets of MDVs with specific functions. One of these subsets would be secreted into the extracellular space, possibly exerting specific extracellular functions rather than serving as mere shuttles of mitochondrial debris outside the cell. In line with this hypothesis, D’Acunzo et al. [14] demonstrated that mitovesicles were not encapsulated by an external membrane, enabling extracellular enzymatic activity of proteins anchored to the outer mitochondrial membrane, such as monoamino oxidase (MAO) A and B, and the capacity to synthesize ATP upon direct contact with adequate substrates.
A role for mitovesicles has been described for the first time in individuals with Down syndrome (DS), in whom the number of brain mitovesicles is greater than and their composition is different from controls [14]. These findings are in keeping with the observation that EVs of neuronal origin retrieved in plasma of individuals with DS have a different cargo molecule repertoire compared with non-DS controls [60]. Mitochondrial dysfunction, ROS overproduction, and mitophagy deficit have been reported in DS [61]. In this context, mitovesicles, like MDVs, may be part of a mitochondrial quality control pathway that contributes to the removal of detrimental mitochondrial material from the cell and mitigates oxidative stress in a homeostatic feedback loop.
Taken as a whole, changes in intracellular organelle homeostasis may be reflected by different levels of mitovesicles and MDVs, and, more in general, in EV populations such as exosomes [62]. Changes in all these shuttling systems, collectively referred to as EVs, can have pivotal roles as modulators of cell-to-cell communication and in remodeling of the extracellular environment. Whether EVs convey beneficial or negative effects to the recipient cells is discussed in the next sections.
4. Mitochondrial-Derived Vesicles and Mitovesicles: Isolation and Characterization Methods
MDVs are a relatively novel aspect of vesicle biology, with limited availability in the literature and knowledge of purification methods. Available techniques for the isolation of secreted MDVs often lead to co-purification of other EV classes, thus making essential their subsequent characterization to distinguish MDV subtypes.
Due to their small size, the isolation of pure EV samples and their quantification are major limitations in the field of EV research. Ultrafiltration, size exclusion chromatography (SEC), precipitation, immunoaffinity-based capture/immunoprecipitation, and ultracentrifugation are current methods for isolation of EV fractions from biological fluids and cellular media [63]. The rapid development of these methods has made isolation procedures easier and faster with larger yields of purified EVs, although disadvantages exist for each of them.
The ultrafiltration method allows EV concentration on pore-containing membranes and purification based on size. With this method, EV purity is moderate, and membrane clogging and EVs trapped in membranes are common [64]. SEC separates macromolecules based on their size by applying a fluid on a column packed with porous, polymeric beads with the advantage of allowing precise separation of both large and small EVs without affecting their structure. However, this approach requires a long runtime [65]. The precipitation method is an easier procedure, but it determines alteration of EV solubility or dispersibility with possible co-precipitation non-EV contaminants, such as proteins and/or polymeric materials [66]. Finally, the immunoaffinity-based capture/immunoprecipitation based on specific interactions between a membrane-bound antigen of EVs and an immobilized antibody allows for isolating highly purified EV fractions with the possibility of subtyping. However, this method requires knowledge of EV tags, and co-purification of different EV subpopulations sharing similar EV tags may occur [67].
The ultracentrifugation method remains the elective approach for the purification of EVs as recommended by the International Society of Extracellular Vesicles (ISEV) [68]. The method is based on density-, size-, and shape-based sequential separation, consisting of several centrifugation steps to remove cells, debris, and, finally, pelleted EVs. This approach is the most used preparative procedure for EV purification and has also been applied for MDV characterization [57]. Via ultracentrifugation, it is possible to obtain a good yield of EVs/MDVs starting from different matrices, such as cell media, plasma, serum, or urine samples. However, there are, also in this case, some caveats. Ultracentrifugation allows purification of heterogenous classes of EVs, among which MDVs are present. The isolation of one type of EV is unfeasible with this method and, after ultracentrifugation, other methods become essential for MDV characterization [3][10][57][69][70]. As per protocol of MDV/EV purification starting from serum/plasma, samples are diluted with equal volumes of phosphate-buffered saline (PBS) before centrifugation to reduce fluid viscosity and subjected to a first centrifugation at 2000× g at 4 °C for 30 min to discard any cell contamination [57]. Supernatants are then centrifuged at 12,000× g at 4 °C for 45 min to remove apoptotic bodies, cell debris, and large vesicles (mean size > 200 nm) and, subsequently, ultracentrifuged at 110,000× g at 4 °C for 2 h. Pellets are recovered and resuspended in PBS, filtered through a 0.22 μm filter, and ultracentrifuged at 110,000× g at 4 °C for 70 min to eliminate contaminant proteins. Pellets enriched in purified EVs, among which MDVs are also included, are resuspended in 100 μL of PBS. To quantify EVs, total protein concentration is measured using the high-sensitivity BCA assay [57].
According to ISEV guidelines, purification methods should be validated by complementary approaches such as electron microscopy analysis (TEM), nanoparticle tracking analysis (NTA), and Western immunoblotting. The latter technique is useful not only to verify the occurrence of positive and negative markers of purification, but also to characterize the protein content of EVs and determine whether the purified populations also contain MDVs [3][10][57][69][70]. This analysis can be performed using specific antibodies that recognize MDV markers such as TOMM20, ATP synthase subunit 5A (ATP5A), mitochondrial cytochrome C oxidase subunit I (MTCOI), NADH:ubiquinone oxidoreductase subunit B8 (NDUFB8), NADH:ubiquinone oxidoreductase core subunit (NDUFS3), succinate dehydrogenase complex flavoprotein subunit A (SDHA), succinate dehydrogenase complex iron–sulfur subunit B (SDHB) and, ubiquinol–cytochrome C reductase core protein 2 (UQCRC2) [57][69][70]. Further confirmation of mitochondrial origin can be obtained via the identification of mtDNA encapsulated within these vesicles [71].
Alternatively, MDVs can be visualized as small vesicular structures that show cargo selectivity under microscopy approaches when using highly specific antibodies against endogenous mitochondrial proteins or a combination of transfected mitochondrial green fluorescent protein (GFP)-tagged constructs with antibodies to label a second or third mitochondrial protein [3]. The absolute dependence on protein specificity and background signals are important limitations related to the use of antibodies. In addition, GFP-tagged and overexpressed proteins are not always efficient for cargo selectivity, perhaps because they are first targeted by proteases. The analyses of mitochondrial proteins and mtDNA content in MDVs, as well as proteomic approaches, remain more relevant analytical tools.
Ultracentrifugation is also a preparative method for subsequent isolation of mitovesicles using a high-resolution density gradient [14][54]. By slight modification of the original sucrose gradient isolation method, D’Acunzo et al. [14][54] isolated and fractioned EVs with an iodixanol-based step gradient density column. They were the first group to develop a method to isolate EVs from murine and human postmortem brains using a sucrose-based step gradient [54][72][73][74]. The method involves a short enzymatic digestion of the brain tissue to loosen the extracellular matrix (ECM), followed by differential centrifugation and a sucrose gradient [75][76]. Because the sucrose-based gradient is hyperosmotic compared with biofluids, this method causes vesicle shrinkage, which may be of concern if biologically active EVs are required for later analyses. Furthermore, it is impossible to generate high-resolution step gradients with sucrose as a density medium with sucrose-based solutions with similar molarities, making the separation of EV subpopulations difficult. As a consequence, the authors modified the fractionation method by using iodixanol to create the density column, demonstrating a successful separation between different subtypes of brain EVs [14][54]. Iodixanol is isosmolar with body fluids across a wide range of dilutions, is inert, has relatively low viscosity, and allows generation of fractions with closer density ranges than sucrose, enabling efficient EV separation with higher resolution power. After enzymatic digestion of tissue, the EV pellet is purified using the ultracentrifugation method described above and, after stratification on iodixanol gradient, fractions containing microvesicles, exosomes, and mitovesicles are obtained. According to ISEV recommendations, the latter can be further analyzed by NTA, electron microscopy, Western blot analysis, and measurement of microvesicular ATP kinetics [14][54].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241813835
References
- Pickles, S.; Vigié, P.; Youle, R. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, R170–R185.
- Fang, E.F.; Palikaras, K.; Sun, N.; Fivenson, E.M.; Spangler, R.D.; Kerr, J.S.; Cordonnier, S.A.; Hou, Y.; Dombi, E.; Kassahun, H.; et al. In vitro and in vivo detection of mitophagy in human cells, C. elegans, and mice. J. Vis. Exp. 2017, 2017, 56301.
- Sugiura, A.; McLelland, G.-L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156.
- McLelland, G.-L.; Soubannier, V.; Chen, C.X.; McBride, H.M.; Fon, E.A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014, 33, 282–295.
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372.
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pépin, G.; Germain, M. Selective Packaging of Mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971.
- Liang, W.; Sagar, S.; Ravindran, R.; Najor, R.H.; Quiles, J.M.; Chi, L.; Diao, R.Y.; Woodall, B.P.; Leon, L.J.; Zumaya, E.; et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 2023, 14, 5031.
- Matheoud, D.; Sugiura, A.; Bellemare-Pelletier, A.; Laplante, A.; Rondeau, C.; Chemali, M.; Fazel, A.; Bergeron, J.J.; Trudeau, L.E.; Burelle, Y.; et al. Parkinson’s disease-related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell 2016, 166, 314–327.
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Romano, R.; Bucci, C.; Marzetti, E. Extracellular vesicles and damage-associated molecular patterns: A Pandora’s box in health and disease. Front. Immunol. 2020, 11, 601740.
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.; Bucci, C.; Marzetti, E. Circulating extracellular vesicles: Friends and foes in neurodegeneration. Neural Regen. Res. 2022, 17, 534–542.
- D’Acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085.
- Popov, L.D. Mitochondrial-derived vesicles: Recent insights. J. Cell. Mol. Med. 2022, 26, 3323–3328.
- Neuspiel, M.; Schauss, A.C.; Braschi, E.; Zunino, R.; Rippstein, P.; Rachubinski, R.A.; Andrade-Navarro, M.A.; McBride, H.M. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr. Biol. 2008, 18, 102–108.
- Schumann, U.; Subramani, S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008, 18, 253–256.
- Andrade-Navarro, M.A.; Sanchez-Pulido, L.; McBride, H.M. Mitochondrial vesicles: An ancient process providing new links to peroxisomes. Curr. Opin. Cell Biol. 2009, 21, 560–567.
- Vasam, G.; Nadeau, R.; Cadete, V.J.J.; Lavallée-Adam, M.; Menzies, K.J.; Burelle, Y. proteomics characterization of mitochondrial-derived vesicles under oxidative stress. FASEB J. 2021, 35, e21278.
- Beveridge, T.J. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733.
- Pérez-Cruz, C.; Carrión, O.; Delgado, L.; Martinez, G.; López-Iglesias, C.; Mercade, E. new type of outer membrane vesicle produced by the gram-negative bacterium Shewanella vesiculosa M7T: Implications for DNA content. Appl. Environ. Microbiol. 2013, 79, 1874–1881.
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. outer-inner membrane vesicles naturally secreted by Gram-negative pathogenic bacteria. PLoS ONE 2015, 10, e0116896.
- Mashburn, L.M.; Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005, 437, 422–425.
- Deatheragea, B.L.; Cooksona, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957.
- Cadete, V.J.J.; Deschênes, S.; Cuillerier, A.; Brisebois, F.; Sugiura, A.; Vincent, A.; Turnbull, D.; Picard, M.; McBride, H.M.; Burelle, Y. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 2016, 594, 5343–5362.
- Yamashita, A.; Fujimoto, M.; Katayama, K.; Yamaoka, S.; Tsutsumi, N.; Arimura, S.I. Formation of mitochondrial outer membrane derived protrusions and vesicles in Arabidopsis thaliana. PLoS ONE 2016, 11, e0146717.
- Lin, M.Y.; Cheng, X.T.; Tammineni, P.; Xie, Y.; Zhou, B.; Cai, Q.; Sheng, Z.H. Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 2017, 94, 595–610.e6.
- Soubannier, V.; Rippstein, P.; Kaufman, B.A.; Shoubridge, E.A.; McBride, H.M. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS ONE 2012, 7, e52830.
- Wang, W.; Wang, X.; Fujioka, H.; Hoppel, C.; Whone, A.L.; Caldwell, M.A.; Cullen, P.J.; Liu, J.; Zhu, X. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 2016, 22, 54–63.
- Abuaita, B.H.; Schultz, T.L.; O’Riordan, M.X. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized Staphylococcus aureus. Cell Host Microbe 2018, 24, 625–636.e5.
- Sugiura, A.; Mattie, S.; Prudent, J.; Mcbride, H.M. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 2017, 542, 251–254.
- Li, B.; Zhao, H.; Wu, Y.; Zhu, Y.; Zhang, J.; Yang, G.; Yan, Q.; Li, J.; Li, T.; Liu, L. Mitochondrial-derived vesicles protect cardiomyocytes against hypoxic damage. Front. Cell Dev. Biol. 2020, 8, 214.
- Pickrell, A.M.; Youle, R.J. The Roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273.
- Pouikli, A.; Parekh, S.; Maleszewska, M.; Nikopoulou, C.; Baghdadi, M.; Tripodi, I.; Folz-Donahue, K.; Hinze, Y.; Mesaros, A.; Hoey, D.; et al. Chromatin remodeling due to degradation of citrate carrier impairs osteogenesis of aged mesenchymal stem cells. Nat. Aging 2021, 1, 810–825.
- Mondet, J.; Lo Presti, C.; Chevalier, S.; Bertrand, A.; Tondeur, S.; Blanchet, S.; Mc Leer, A.; Pernet-Gallay, K.; Mossuz, P. Mitochondria in human acute myeloid leukemia cell lines have ultrastructural alterations linked to deregulation of their respiratory profiles. Exp. Hematol. 2021, 98, 53–62.e3.
- Soubannier, V.; McLelland, G.-L.; Zunino, R.; Braschi, E.; Rippstein, P.; Fon, E.A.; McBride, H.M. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 2012, 22, 135–141.
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15.
- Greene, A.; Grenier, K.; Aguileta, M.; Muise, S.; Farazifard, R.; Haque, M.; McBride, H.; Park, D.; Fon, E. Mitochondrial processing peptidase regulates PINK1 processing, import and pParkin recruitment. EMBO Rep. 2012, 13, 378–385.
- Yamano, K.; Youle, R.J. PINK1 is degraded through the N-end rule pathway. Autophagy 2013, 9, 1758–1769.
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298.
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.-S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221.
- Kucera, A.; Bakke, O.; Progida, C. The multiple roles of Rab9 in the endolysosomal system. Commun. Integr. Biol. 2016, 9, e1204498.
- Soulet, F.; Yarar, D.; Leonard, M.; Schmid, S.L. SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol. Biol. Cell 2005, 16, 2058–2067.
- Roberts, R.F.; Fon, E.A. Presenting mitochondrial antigens: PINK1, Parkin and MDVs steal the show. Cell Res. 2016, 26, 1180–1181.
- Xu, Y.; Shen, J.; Ran, Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 2020, 16, 3–17.
- Zeng, X.; Li, X.; Zhang, Y.; Cao, C.; Zhou, Q. IL6 induces MtDNA leakage to affect the immune escape of endometrial carcinoma via CGAS-STING. J. Immunol. Res. 2022, 2022, 3815853.
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernández-Delgado, I.; Latorre-Pellicer, A.; Acín-Pérez, R.; Martín-Cófreces, N.B.; Jaso-Tamame, Á.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658.
- Ko, J.H.; Kim, H.J.; Jeong, H.J.; Lee, H.J.; Oh, J.Y. Mesenchymal stem and stromal cells harness macrophage-derived amphiregulin to maintain tissue homeostasis. Cell Rep. 2020, 30, 3806–3820.e6.
- Ramirez, A.; Old, W.; Selwood, D.L.; Liu, X. Cannabidiol activates PINK1-Parkin-dependent mitophagy and mitochondrial-derived vesicles. Eur. J. Cell Biol. 2022, 101, 151185.
- König, T.; Nolte, H.; Aaltonen, M.J.; Tatsuta, T.; Krols, M.; Stroh, T.; Langer, T.; McBride, H.M. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat. Cell Biol. 2021, 23, 1271–1286.
- Braschi, E.; Goyon, V.; Zunino, R.; Mohanty, A.; Xu, L.; McBride, H.M. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 2010, 20, 1310–1315.
- Ryan, T.A.; Phillips, E.O.; Collier, C.L.; JB Robinson, A.; Routledge, D.; Wood, R.E.; Assar, E.A.; Tumbarello, D.A. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020, 39, e102539.
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bossola, M.; Landi, F.; Bernabei, R.; Bucci, C.; Marzetti, E. Generation and release of mitochondrial-derived vesicles in health, aging and disease. J. Clin. Med. 2020, 9, 1440.
- D’Acunzo, P.; Kim, Y.; Ungania, J.M.; Pérez-González, R.; Goulbourne, C.N.; Levy, E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat. Protoc. 2022, 17, 2517–2549.
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89.
- Fecher, C.; Trovò, L.; Müller, S.A.; Snaidero, N.; Wettmarshausen, J.; Heink, S.; Ortiz, O.; Wagner, I.; Kühn, R.; Hartmann, J.; et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 2019, 22, 1731–1742.
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial-derived vesicles as candidate biomarkers in Parkinson’s disease: Rationale, design and methods of the EXosomes in PArkiNson Disease (EXPAND) study. Int. J. Mol. Sci. 2019, 20, 2373.
- Stevic, I.; Buescher, G.; Ricklefs, F.L. Monitoring therapy efficiency in cancer through extracellular vesicles. Cells 2020, 9, 130.
- Guerra, F.; Paiano, A.; Migoni, D.; Girolimetti, G.; Perrone, A.M.; De Iaco, P.; Fanizzi, F.P.; Gasparre, G.; Bucci, C. Modulation of RAB7A protein expression determines resistance to cisplatin through late endocytic pathway impairment and extracellular vesicular secretion. Cancers 2019, 11, 52.
- Perluigi, M.; Picca, A.; Montanari, E.; Calvani, R.; Marini, F.; Matassa, R.; Tramutola, A.; Villani, A.; Familiari, G.; Di Domenico, F.; et al. Aberrant crosstalk between insulin signaling and MTOR in young Down syndrome individuals revealed by neuronal-derived extracellular vesicles. Alzheimers Dement. 2022, 18, 1498–1510.
- Bordi, M.; Darji, S.; Sato, Y.; Mellén, M.; Berg, M.J.; Kumar, A.; Jiang, Y.; Nixon, R.A. mTOR hyperactivation in Down syndrome underlies deficits in autophagy induction, autophagosome formation, and mitophagy. Cell Death Dis. 2019, 10, 563.
- Mathews, P.M.; Levy, E. Exosome production is key to neuronal endosomal pathway integrity in neurodegenerative diseases. Front. Neurosci. 2019, 13, 1347.
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating methods for isolation and quantification of exosomes: A review. Mol. Biotechnol. 2021, 63, 249–266.
- Barreiro, K.; Huber, T.B.; Holthofer, H. Isolating urinary extracellular vesicles as biomarkers for diabetic disease. Methods Mol. Biol. 2020, 2067, 175–188.
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via MiR-22. PLoS ONE 2014, 9, e88685.
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 2016, 6, 23978.
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Picca, A.; Beli, R.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Bucci, C.; Guerra, F.; Marzetti, E. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells 2020, 9, 973.
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial signatures in circulating extracellular vesicles of older adults with Parkinson’s disease: Results from the EXosomes in PArkiNson’s Disease (EXPAND) study. J. Clin. Med. 2020, 9, 504.
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075.
- Perez-Gonzalez, R.; Gauthier, S.A.; Kumar, A.; Levy, E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. 2012, 287, 43108–43115.
- Pérez-González, R.; Gauthier, S.A.; Sharma, A.; Miller, C.; Pawlik, M.; Kaur, G.; Kim, Y.; Levy, E. A pleiotropic role for exosomes loaded with the amyloid β precursor protein carboxyl-terminal fragments in the brain of Down syndrome patients. Neurobiol. Aging 2019, 84, 26–32.
- Pérez-González, R.; Gauthier, S.A.; Kumar, A.; Saito, M.; Saito, M.; Levy, E. A method for isolation of extracellular vesicles and characterization of exosomes from brain extracellular space. Methods Mol. Biol. 2017, 1545, 139–151.
- Vella, L.J.; Scicluna, B.J.; Cheng, L.; Bawden, E.G.; Masters, C.L.; Ang, C.S.; Willamson, N.; McLean, C.; Barnham, K.J.; Hill, A.F. A rigorous method to enrich for exosomes from brain tissue. J. Extracell. Vesicles 2017, 6, 1348885.
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580.
This entry is offline, you can click here to edit this entry!
