Ascorbic acid (AA), or vitamin C, is one of the most important vitamins consumed through the diet due to its critical role in many biological processes. Although the human body cannot synthesize it, AA is essential in maintaining healthy bodily structure, acting as a cofactor of many enzymes involved in collagen synthesis and an efficient immune system. At the same time, AA is used in the cosmetic field for its antioxidant and antipigmentary properties, in the food industry as additive, and in chemical synthesis as reducing agent. AA can be chemically synthesized, produced by the oxidative fermentation of bacteria, or extracted from natural sources.
- ascorbic acid
- vitamin C
- dietary
- antioxidant
- cosmetics
- additives
1. Introduction
2. AA as Dietary Supplement
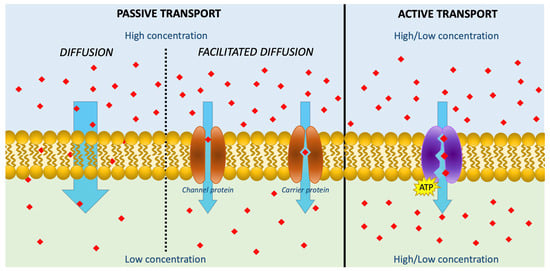
3. AA in Cosmetics
4. AA Industrial Applications
This entry is adapted from the peer-reviewed paper 10.3390/pr11113167
References
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182.
- Iqbal, K.; Khan, A.; Khattak, M. Biological significance of ascorbic acid (vitamin C) in human health–A review. Pak. J. Nutr. 2004, 3, 5–13.
- Mazurek, A.; Włodarczyk-Stasiak, M. A New Method for the Determination of Total Content of Vitamin C, Ascorbic and Dehydroascorbic Acid, in Food Products with the Voltammetric Technique with the Use of Tris(2-carboxyethyl)phosphine as a Reducing Reagent. Molecules 2023, 28, 812.
- Tucaliuc, A.; Cîșlaru, A.; Kloetzer, L.; Blaga, A.C. Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C. Processes 2022, 10, 1595.
- Lim, S.M.; Lau, M.S.L.; Tiong, E.I.J.; Goon, M.M.; Lau, R.J.C.; Yeo, W.S.; Lau, S.Y.; Mubarak, N.M. Process design and economic studies of two-step fermentation for production of ascorbic acid. SN Appl. Sci. 2020, 2, 816.
- Yang, W.; Xu, H. Industrial Fermentation of Vitamin C. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Wiley: Hoboken, NJ, USA, 2016; pp. 161–192.
- Zhou, M.; Bi, Y.; Ding, M.; Yuan, Y. One-Step Biosynthesis of Vitamin C in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 643472.
- Wang, P.; Zeng, W.; Xu, S.; Du, G.; Zhou, J.; Chen, J. Current challenges facing one-step production of l-ascorbic acid. Biotechnol. Adv. 2018, 36, 1882–1899.
- Pappenberger, G.; Hohmann, H.-P. Industrial Production of l-Ascorbic Acid (Vitamin C) and d-Isoascorbic Acid. In Biotechnology of Food and Feed Additives; Zorn, H., Czermak, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–188.
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615.
- Cotruţ, R.; Bădulescu, L. UPLC Rapid Quantification of Ascorbic Acid in Several Fruits and Vegetables Extracted Using Different Solvents. Agric. Agric. Sci. Procedia 2016, 10, 160–166.
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963.
- FAO Joint; World Health Organization. Human Vitamin and Mineral Requirements; WHO: Geneva, Switzerland, 2002.
- Rivas, M.Á.; Casquete, R.; Martín, A.; Córdoba, M.d.G.; Aranda, E.; Benito, M.J. Strategies to Increase the Biological and Biotechnological Value of Polysaccharides from Agricultural Waste for Application in Healthy Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 5937.
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Namdar, B.; Akbarmivehie, M.; Mirsaeedghazi, H.; Azarikia, F. Optimization of ultrasonic-assisted extraction of phenolic compounds from pomegranate and orange peels and their antioxidant activity in a functional drink. Food Biosci. 2022, 49, 101918.
- Abdurrahman Isa, A.; Samsuri, S.; Aini Amran, N. Integration of Maceration and Freeze Concentration for Recovery of Vitamin C from Orange Peel Waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012101.
- Arora, M.; Kaur, P. Phytochemical screening of orange peel and pulp. Int. J. Res. Eng. Technol. 2013, 2, 517–522.
- Roussos, P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (L.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hortic. 2011, 129, 253–258.
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926.
- Sharma, K.; Akansha, C.E. Comparative studies of proximate, mineral and phytochemical compositions of pomegranate (Punica granatum) in peel, seed and whole fruit powder. Methods 2018, 17, 18.
- Marra, F.; Petrovicova, B.; Canino, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Pomegranate Wastes Are Rich in Bioactive Compounds with Potential Benefit on Human Health. Molecules 2022, 27, 5555.
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martínez, J.; Godoy, H.T. Vitamin C in camu-camu : Evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166.
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378.
- Mittu, B.; Bhat, Z.R.; Chauhan, A.; Kour, J.; Behera, A.; Kaur, M. Chapter 16—Ascorbic acid. In Nutraceuticals and Health Car; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 289–302.
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008.
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412.
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755.
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263.
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029.
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58.
- Dresen, E.; Lee, Z.-Y.; Hill, A.; Notz, Q.; Patel, J.J.; Stoppe, C. History of scurvy and use of vitamin C in critical illness: A narrative review. Nutr. Clin. Pract. 2023, 38, 46–54.
- Granger, M.; Eck, P. Chapter Seven—Dietary Vitamin C in Human Health. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 281–310.
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal Dehydroascorbic Acid (DHA) Transport Mediated by the Facilitative Sugar Transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101.
- Frikke-Schmidt, H.; Tveden-Nyborg, P.; Lykkesfeldt, J. l-dehydroascorbic acid can substitute l-ascorbic acid as dietary vitamin C source in guinea pigs. Redox Biol. 2016, 7, 8–13.
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454.
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501.
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43.
- Baron, J.H. Sailors’ scurvy before and after James Lind–A reassessment. Nutr. Rev. 2009, 67, 315–332.
- Ceglie, G.; Macchiarulo, G.; Marchili, M.R.; Marchesi, A.; Rotondi Aufiero, L.; Di Camillo, C.; Villani, A. Scurvy: Still a threat in the well-fed first world? Arch. Dis. Child. 2019, 104, 381.
- Padayatty, S.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016, 22, 463–493.
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029.
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M. Evaluation of prooxidant properties of ascorbic acid. Biophysics 2013, 58, 453–456.
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211.
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020, 72, 1517–1528.
- Carr, A.C.; Rowe, S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 2020, 12, 3286.
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282.
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxidative Med. Cell. Longev. 2019, 2019, 7286737.
- Van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977.
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894.
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706.
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79.
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707.
- Bedhiafi, T.; Inchakalody, V.P.; Fernandes, Q.; Mestiri, S.; Billa, N.; Uddin, S.; Merhi, M.; Dermime, S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022, 146, 112553.
- Kuhn, S.-O.; Meissner, K.; Mayes, L.M.; Bartels, K. Vitamin C in sepsis. Curr. Opin. Anesthesiol. 2018, 31, 55.
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020, 12, 292.
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866.
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., III; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584.
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18, S6–S11.
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of vitamin C in skin diseases. Front. Physiol. 2018, 9, 819.
- Shibuya, S.; Sakaguchi, I.; Ito, S.; Kato, E.; Watanabe, K.; Izuo, N.; Shimizu, T. Topical Application of Trisodium Ascorbyl 6-Palmitate 2-Phosphate Actively Supplies Ascorbate to Skin Cells in an Ascorbate Transporter-Independent Manner. Nutrients 2017, 9, 645.
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702.
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663.
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153.
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The Use of Ascorbic Acid as a Food Additive: Technical-Legal Issues. Ital. J. Food Saf. 2016, 5, 4313.
- Final Report of the Safety Assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as Used in Cosmetics1. Int. J. Toxicol. 2005, 24, 51–111.
- Hieu, T.V.; Guntoro, B.; Qui, N.H.; Quyen, N.T.K.; Hafiz, F.A.A. The application of ascorbic acid as a therapeutic feed additive to boost immunity and antioxidant activity of poultry in heat stress environment. Vet. World 2022, 15, 685–693.
- Hassan, F.A.; Shalaby, A.G.; Elkassas, N.E.M.; El-Medany, S.A.; Hamdi Rabie, A.; Mahrose, K.; Abd El-Aziz, A.; Bassiony, S. Efficacy of ascorbic acid and different sources of orange peel on growth performance, gene expression, anti-oxidant status and microbial activity of growing rabbits under hot conditions. Anim. Biotechnol. 2023, 34, 2480–2491.
- Ostrenko, K.; Nekrasov, R.; Ovcharova, A.; Lemiasheuski, V.; Kutin, I. The Effect of Lithium Salt with Ascorbic Acid on the Antioxidant Status and Productivity of Gestating Sows. Animals 2022, 12, 915.
- Cao, F.; Tian, W.; Wang, M.; Wang, M.; Li, L. Stability enhancement of lead-free CsSnI3 perovskite photodetector with reductive ascorbic acid additive. InfoMat 2020, 2, 577–584.
- Cai, Z.; Li, H.; Wu, J.; Zhu, L.; Ma, X.; Zhang, C. Ascorbic acid stabilised copper nanoclusters as fluorescent sensors for detection of quercetin. RSC Adv. 2020, 10, 8989–8993.
- Sun, K.; Qiu, J.; Liu, J.; Miao, Y. Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. J. Mater. Sci. 2009, 44, 754–758.
- Palomba, M.; Carotenuto, G.; Longo, A. A Brief Review: The Use of L-Ascorbic Acid as a Green Reducing Agent of Graphene Oxide. Materials 2022, 15, 6456.
- Lie, J.; Liu, J.-C. Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid. Sep. Purif. Technol. 2021, 266, 118458.
- Chen, D.; Rao, S.; Wang, D.; Cao, H.; Xie, W.; Liu, Z. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid- l-ascorbic acid system. Chem. Eng. J. 2020, 388, 124321.
