Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Developmental dysplasia of the hip (DDH) is a disorder characterized by abnormal hip development that frequently manifests in infancy and early childhood. Preventing DDH from occurring relies on a timely and accurate diagnosis, which requires careful assessment by medical specialists during early X-ray scans.
- development dysplasia of the hip (DDH)
- deep learning
- acetabular index
- Mask-RCNN
1. Introduction
Developmental dysplasia of the hip (DDH) is the most common musculoskeletal developmental disorder that affects newborns and is regarded as the primary cause of around 25% to 43% of end-stage hip arthroplasty cases [1][2]. DDH poses a significant risk to the quality of life for patients from an early age and could persist into adulthood without proper treatment [3]. Failure to recognize and diagnose DDH in its early stages can lead to functional impairment, chronic hip pain, progressive hip degeneration, and accelerated osteoarthritis that necessitates surgical intervention later in life [4].
DDH occurs when the femoral head does not fit into the socket due to an abnormal hip joint, leading to limited hip range of motion, and noticeable gait abnormalities. Early identification through reliable hip diagnostic screening is crucial for timely intervention [5]. Detecting DDH at an early age significantly reduces risks of long-term complications.
The current clinical approach for diagnosing DDH involves manual measurements of various anatomical characteristics from pelvic radiographs, followed by assessment by experienced clinicians or radiologists. However, this process lacks standardization and heavily relies on subjective metrics, demanding a greater degree of knowledge and clinical experience. Recent advancement in computer-aided medical diagnosis (CAD) supplemented with pattern recognition techniques have made it feasible to incorporate these technologies into practical solutions that offer an automated, standardized, and reliable diagnosis of DDH in infants. These solutions utilize the acetabular index angle measurement (AcI) as a clinically established radiographic measurement for assessing the severity of DDH in individual cases (Figure 1). AcI is measured by calculating the angle between two lines. The first line extends from the medial edge to the most lateral aspect of the acetabular sourcil, which refers to the inner rim of the acetabulum. The second line, known as Hilgenreiner’s line, or H-line, runs horizontally on the pelvis. The degree of the angle formed by their intersection indicates the range within which the acetabular index falls. An increased acetabular inclination range is indicative of the presence of DDH [6].
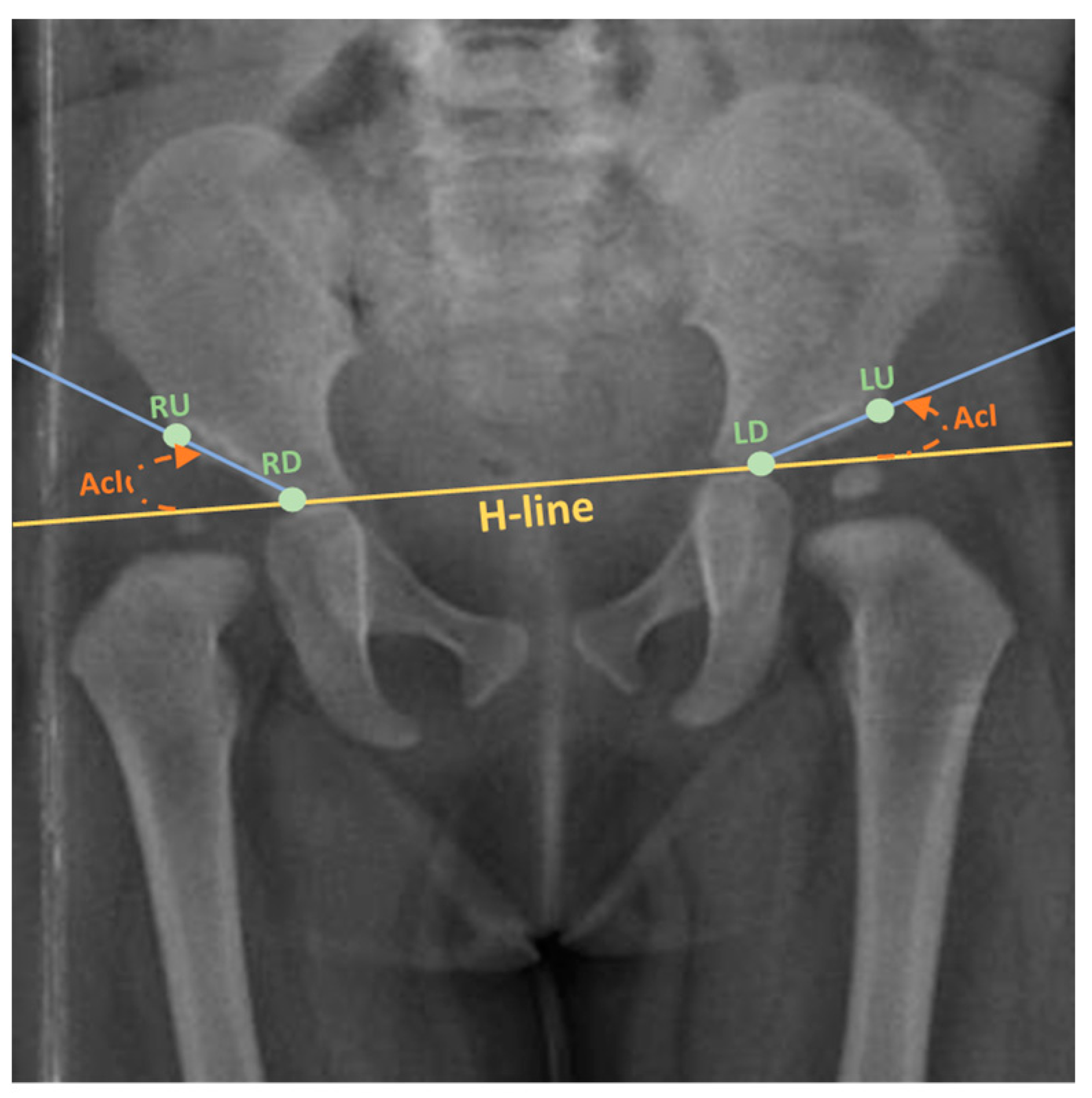
Figure 1. Calculating the AcI, with H-line displayed (Adapted from [7]). Keypoints: LU: Left upper; LD: Left down; RU: Right upper; RD: Right down.
2. Related Literature
There have already been several advances in utilizing CAD to assess DDH in the last decades. The first attempt to automatically detect relevant landmarks from medical images of the infant pelvis could be traced back to 1997, when Overhoff et al. [8], introduced an image processing-based approach to determine the region of interest (ROI) of the acetabulum and approximate the sphere of the femoral head in 3D ultrasound images. The findings of the study were further extended [9] to detect the acetabular landmarks used to assess the relevant angle measurements. More image processing-based algorithms such as the Hough transform were proposed [10] to offer a fully automated approach for the measurement of the acetabular cartilage in MRI medical images. Moreover, in a more recent study, Hough transform was paired with canny edge detection to assess relevant landmarks on the pelvic X-ray images [11].
2.1. Detection & Diagnosis Software Tools
Many medical professionals use CAD-based tools to make assessments in clinical settings for DDH diagnosis and AcI quick measurement. Acetabular Index, an application for portable devices, calculates the index via manually detected landmarks. It measures angles in X-ray images through the aid of a circular transparent template, where the points of interest are marked accurately. The lines automatically formed between points serve to measure the angles of interest. Although an abundance of research attempts to propose a reliable method for automating medical diagnosis, few are deployed and utilized in professional environments. BoneView is a good representation of a fully-fledged medical software powered by AI that offers diagnostic features for bone lesions, fractures, and dislocations detection for bone trauma X-rays. Techniques used in BoneView include a ‘Detectron 2’-based diffusion-convolutional neural network (DCNN) model developed for image training. In addition, it applies natural language processing (NLP) algorithms to infer diagnostic interpretations from radiologists’ reports [12]. Similarly, ImageBiopsy Lab launched several software products to detect anomalies in the musculoskeletal system. One product is IB Lab LAMA for automating the assessment of pre-and post-total knee arthroplasty (TKA) lower limb alignment using AI methodologies in addition to “HIPPO” for hip angles and morphology assessments [13][14][15][16][17].
2.2. Research Utilizing ML/DL-Based Methodologies
Although traditional image processing-based CAD approaches have achieved acceptable results, they struggle upon implementation with larger and more diverse datasets. In addition, they have a lengthy processing procedure that is impractical in medical environments. That is where artificial intelligence (AI) concepts such as machine learning (ML) and deep learning (DL) come into the picture with a variety of algorithms and frameworks that had been implemented for several purposes such as classification, object detection, instance segmentation, and keypoint detection. Several ML- and DL-based solutions have been utilized in anatomical landmark detection and segmentation in the medical X-ray imaging of the pelvis. Thompson et al. [18] utilized a random-forest-based method for measuring the radiograph’s angular parameters (AcI, RMP) based on automatically detected anatomical landmarks. The proposed system was tested and validated on a clinical dataset of 200 cases. Another ML model was proposed by Jiang et al. [19] using a computer-aided system (CAD) to semi-automatically measure the Tönnis angle, Sharp angle, and CE angle following the bone contours of the hip joint. The CAD system consists of four stages that involve manual bone outlining, automatic landmarks extraction, automatic angle measurement, and hip development assessment and classification using a machine-learning modal support vector machine (SVM). The dataset consisted of 248 X-ray images from Zhongshan Hospital of Dalian University, China.
Using a dataset of 9369 images for patients aged from 0.1 to 14 years, Jingyuan Xu et al. [20] used an hourglass network with an encoder–decoder architecture to generate the heatmap for landmark detection of the pelvic images. The landmarks detected were then used to calculate the acetabular index angle and the age of the femoral head. Similarly, Li Q et al. [21] utilized 11,574 orthotopic anterior pelvic X-ray images to train and test a deep-learning model that consists of a modified Mask R-CNN built on FPN and ResNet101. The model detects four key points of sharp angles from pelvic X-ray images and is later used to automate the calculation of the acetabular angle. Mask RCNN architecture has yet another use case where it was implemented by Xu et al. [22] for image segmentation of the pelvis as the first part of a three-stage pipeline. The second stage was built using HRNet to extract the four relevant DDH landmarks. The final stage was a ResNet model providing a binary diagnosis, which was compared with the judgment of three surgeons to evaluate the AI performance on a dataset of 1398 cases. Additionally, Lee et al. [23] used a Mask RCNN model on ultrasound images to segment the shapes formed by the ilium and acetabular head, then assessed two points on the ilium and three points for calculating the α and β, angles using a dataset of 321 ultrasound scans. Zhang et al. [24] built an FR-DDH deep-learning network with baseline of ResNet101 model to extract feature maps and generate potential neighborhood regions to assess the relevant landmarks and calculate the necessary measurements. The study used 10,219 anteroposterior pelvic radiographs from children aged 10 days to 10 years with a mean age of 1.5 years.
Several research in the literature aimed to implement a classification model for diagnosing DDH directly. For instance, in a similar study by [25], authors utilized 13 deep learning CNN pre-trained models to individually perform binary classification of DDH with different input sizes, widths, and depths using the ImageNet dataset fine-tuned on a custom dataset with a size of 354 images. Out of the 13 pre-trained deep-transfer models, DarkNet53 achieved the highest performance with an overall accuracy of 96.3%, equating to 95%, 90.6%, 100% and 94.3% for the F1 score, precision, sensitivity, and specificity, respectively. Park et al. [26] constructed a dataset of 5076 cropped unilateral hip joint images and developed a customized CNN binary classifier to evaluate the abnormality of the extracted unilateral hip joints from AP radiographs.
Similarly, in [27] authors developed an AI-based clinical decision support system in association with non-expert clinical DDH ultrasound staff. In the workflow experiment, the AI-based app exhibited 100% specificity and recommended follow-ups with relatively fewer errors. In [28], authors presented a novel deep learning-based approach to DDH diagnosis by misshapen pelvis landmark detection using local–global feature learning. The technique was name as Pyramid Non-local UNet (PN-UNet). The scheme exhibited good average point-to-point error. In this regard, a self-created dataset comprising 10,000 X-ray images was investigated. Earlier, the same authors presented a similar work on the same dataset but using spatial local correlation mining with CNN. Nonetheless, the later scheme was better in terms of mean absolute error (MAE) [29].
Such types of automated and AI-based studies have been promising in contrast to the general practitioners’ assessments and surveillance which requires mass screening which is laborious as well as erroneous [30].
Likewise, there are several applications of numerical and deep-learning approaches to solve problems in interdisciplinary areas such as physics [31], hyperphysical [32] and healthcare sectors [33][34][35].
This entry is adapted from the peer-reviewed paper 10.3390/jimaging9110242
References
- Gambling, T.S.; Long, A. Psycho-social impact of developmental dysplasia of the hip and of differential access to early diagnosis and treatment: A narrative study of young adults. SAGE Open Med. 2019, 7, 2050312119836010.
- Pun, S. Hip dysplasia in the young adult caused by residual childhood and adolescent-onset dysplasia. Curr. Rev. Musculoskelet. Med. 2016, 9, 427–434.
- Sadat-Ali, M. Developmental Dysplasia of the Hip (DDH) in Saudi Arabia: Time to Wake up. A Systematic Review (1980–2018). Open J. Epidemiol. 2020, 10, 125.
- Nandhagopal, T.; De Cicco, F.L. Developmental Dysplasia of the Hip–NCBI Bookshelf; StatPearls: St. Petersburg, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK563157/ (accessed on 25 September 2022).
- AAOS. Developmental Dislocation (Dysplasia) of the Hip (DDH). 2022. Available online: https://orthoinfo.aaos.org/en/diseases--conditions/developmental-dislocation-dysplasia-of-the-hip-ddh/ (accessed on 28 August 2023).
- Chmiel-Nowak, M. Acetabular Index. Radiology Reference Article. 2022. Available online: https://radiopaedia.org/articles/acetabular-index?lang=gb (accessed on 6 October 2022).
- Fraiwan, M.; Al-Kofahi, N.; Hanatleh, O.; Ibnian, A. A Dataset of DDH X-ray Images. Available online: https://data.mendeley.com/datasets/jf3pv98m9g/2 (accessed on 15 June 2023).
- Overhoff, H.M.; Lazovic, D.; Franke, J.; Jan, U.V. Automatic determination of the newborn’s femoral head from three-dimensional ultrasound image data. In Lecture Notes in Computer Science; Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; Springer: Berlin/Heidelberg, Germany, 1997.
- Overhoff, H.M.; Lazovic, D.; von Jan, U.; Heinze, P. Computer-based determination of the newborn’s femoral head coverage using three-dimensional ultrasound scans. In Lecture Notes in Computer Science; Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; Springer: Berlin/Heidelberg, Germany, 1998.
- Nishii, T.; Sugano, N.; Sato, Y.; Tanaka, H.; Miki, H.; Yoshikawa, H. Three-dimensional distribution of acetabular cartilage thickness in patients with hip dysplasia: A fully automated computational analysis of MR imaging. Osteoarthr. Cartil. 2004, 12, 650–657.
- Al-Bashir, A.K.; Al-Abed, M.; Sharkh, F.M.A.; Kordeya, M.N.; Rousan, F.M. Algorithm for automatic angles measurement and screening for Developmental Dysplasia of the Hip (DDH). In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015.
- BoneView—GLEAMER—Medical-Grade AI for Radiology. GLEAMER—Medical-Grade AI for Radiology. 2022. Available online: https://www.gleamer.ai/solutions/boneview/ (accessed on 4 October 2022).
- Carlisle, J.C.; Zebala, L.P.; Shia, D.S.; Hunt, D.; Morgan, P.M.; Prather, H.; Wright, R.W.; Steger-May, K.; Clohisy, J.C. Reliability of various observers in determining common radiographic parameters of adult hip structural anatomy. Iowa Orthop. J. 2011, 31, 52–58.
- Nguyen, T.; Maarek, R.; Hermann, A.-L.; Kammoun, A.; Marchi, A.; Khelifi-Touhami, M.R.; Collin, M.; Jaillard, A.; Kompel, A.J.; Hayashi, D.; et al. Assessment of an artificial intelligence aid for the detection of appendicular skeletal fractures in children and young adults by senior and junior radiologists. Pediatr. Radiol. 2022, 52, 2215–2226.
- IB Lab LAMA CE. 2022. Available online: https://www.imagebiopsy.com/product/lama-ce?utm_lang=en (accessed on 4 October 2022).
- IB Lab HIPPO CE. 2022. Available online: https://www.imagebiopsy.com/product/hippo-ce?utm_lang=en (accessed on 4 October 2022).
- ImageBiopsy Lab—Products CE, FDA. 2022. Available online: https://www.imagebiopsy.com/products?utm_lang=en (accessed on 4 October 2022).
- Thompson, P.; Perry, D.C.; Cootes, T.F.; Lindner, C. Automation of clinical measurements on radiographs of children’s hips. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2022 25th International Conference, Singapore, 18–22 September 2022.
- Jiang, Y.; Yang, G.; Liang, Y.; Shi, Q.; Cui, B.; Chang, X.; Qiu, Z.; Zhao, X. Computer-Aided System Application Value for Assessing Hip Development. Front. Physiol. 2020, 11, 587161.
- Xu, J.; Xie, H.; Tan, Q.; Wu, H.; Liu, C.; Zhang, S.; Mao, Z.; Zhang, Y. Multi-task hourglass network for online automatic diagnosis of developmental dysplasia of the hip. World Wide Web 2022, 26, 539–559.
- Li, Q.; Zhong, L.; Huang, H.; Liu, H.; Qin, Y.; Wang, Y.; Zhou, Z.; Liu, H.; Yang, W.; Qin, M.; et al. Auxiliary diagnosis of developmental dysplasia of the hip by automated detection of Sharp’s angle on standardized anteroposterior pelvic radiographs. Medicine 2019, 98, e18500.
- Xu, W.; Shu, L.; Gong, P.; Huang, C.; Xu, J.; Zhao, J.; Shu, Q.; Zhu, M.; Qi, G.; Zhao, G.; et al. A Deep-Learning Aided Diagnostic System in Assessing Developmental Dysplasia of the Hip on Pediatric Pelvic Radiographs. Front. Pediatr. 2022, 9, 785480.
- Lee, J.-H. Sensors & Transducers the Novel Computer Aided Diagnostic System on Medical Images for Parameter Calculation. 2021. Available online: http://www.sensorsportal.com (accessed on 30 January 2023).
- Zhang, S.C.; Sun, J.; Liu, C.B.; Fang, J.H.; Xie, H.T.; Ning, B. Clinical application of artificial intelligence-assisted diagnosis using anteroposterior pelvic radiographs in children with developmental dysplasia of the hip. Bone Jt. J. 2020, 102, 1574–1581.
- Fraiwan, M.; Al-Kofahi, N.; Ibnian, A.; Hanatleh, O. Detection of developmental dysplasia of the hip in X-ray images using deep transfer learning. BMC Med. Inform. Decis. Mak. 2022, 22, 216.
- Park, H.S.; Jeon, K.; Cho, Y.J.; Kim, S.W.; Lee, S.B.; Choi, G.; Lee, S.; Choi, Y.H.; Cheon, J.-E.; Kim, W.S.; et al. Diagnostic performance of a new convolutional neural network algorithm for detecting developmental dysplasia of the hip on anteroposterior radiographs. Korean J. Radiol. 2021, 22, 612–623.
- Jaremko, J.L.; Hareendranathan, A.; Bolouri, S.E.S.; Frey, R.F.; Dulai, S.; Bailey, A.L. AI aided workflow for hip dysplasia screening using ultrasound in primary care clinics. Sci. Rep. 2023, 13, 9224.
- Liu, C.; Xie, H.; Zhang, S.; Mao, Z.; Sun, J.; Zhang, Y. Misshapen Pelvis Landmark Detection with Local-Global Feature Learning for Diagnosing Developmental Dysplasia of the Hip. IEEE Trans. Med. Imaging 2020, 39, 3944–3954.
- Liu, C.; Xie, H.; Zhang, S.; Xu, J.; Sun, J.; Zhang, Y. Misshapen Pelvis Landmark Detection by Spatial Local Correlation Mining for Diagnosing Developmental Dysplasia of the Hip. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2019: 22nd International Conference, Shenzhen, China, 13–17 October 2019; Proceedings, Part VI. Springer: Berlin/Heidelberg, Germany, 2019; pp. 441–449.
- Williams, N. Improving early detection of developmental dysplasia of the hip through general practitioner assessment and surveillance. Aust. J. Gen. Pract. 2018, 47, 619–623.
- Rahman, A. Memetic computing based numerical solution to Troesch problem. J. Intell. Fuzzy Syst. 2019, 37, 1545–1554.
- Musleh, D.; Alotaibi, M.; Alhaidari, F.; Rahman, A.; Mohammad, R.M. Intrusion Detection System Using Feature Extraction with Machine Learning Algorithms in IoT. J. Sens. Actuator Netw. 2023, 12, 29.
- Den, H.; Ito, J.; Kokaze, A. Diagnostic accuracy of a deep learning model using YOLOv5 for detecting developmental dysplasia of the hip on radiography images. Sci. Rep. 2023, 13, 6693.
- Nasir, M.U.; Khan, S.; Mehmood, S.; Khan, M.A.; Rahman, A.-U.; Hwang, S.O. IoMT-Based Osteosarcoma Cancer Detection in Histopathology Images Using Transfer Learning Empowered with Blockchain, Fog Computing, and Edge Computing. Sensors 2022, 22, 5444.
- Rahman, A.U.; Nasir, M.U.; Gollapalli, M.; Zubair, M.; Saleem, M.A.; Mehmood, S.; Khan, M.A.; Mosavi, A. Advance Genome Disorder Prediction Model Empowered with Deep Learning. IEEE Access 2022, 10, 70317–70328.
This entry is offline, you can click here to edit this entry!
