The foot and ankle serve vital roles in weight bearing, balance, and flexibility but are susceptible to many diverse ailments, making treatment difficult. More commonly, Total Ankle Arthroplasty (TAA) and Total Talus Replacement (TTR) are used for patients with ankle degeneration and avascular necrosis of the talus, respectively. Ankle prosthesis and orthosis are also indicated for use with lower limb extremity amputations or locomotor disability, leading to the development of powered exoskeletons. However, patient outcomes remain suboptimal, commonly due to the misfitting of implants to the patient-specific anatomy. Additive manufacturing (AM) is being used to create customized, patient-specific implants and porous implant cages that provide structural support while allowing for increased bony ingrowth and to develop customized, lightweight exoskeletons with multifunctional actuators. AM implants and devices have shown success in preserving stability and mobility of the joint and achieving fast recovery, as well as significant improvements in gait rehabilitation, gait assistance, and strength for patients.
- additive manufacturing
- 3D printing
- total ankle arthroplasty
- total talus arthroplasty
- ankle prosthesis
1. Introduction
2. Total Ankle Replacement
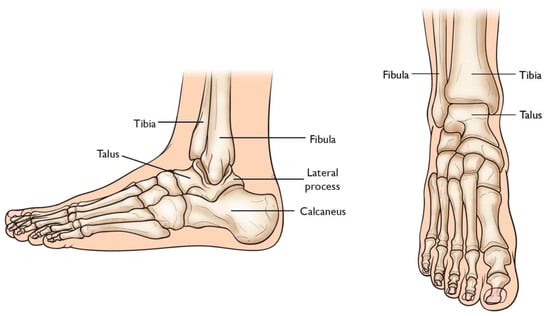
2.1. The Evolution of Total Ankle Arthroplasty Implants
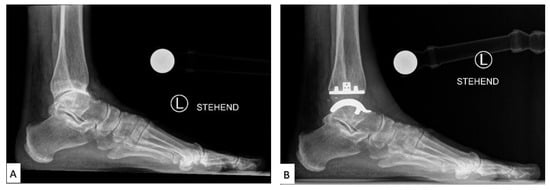
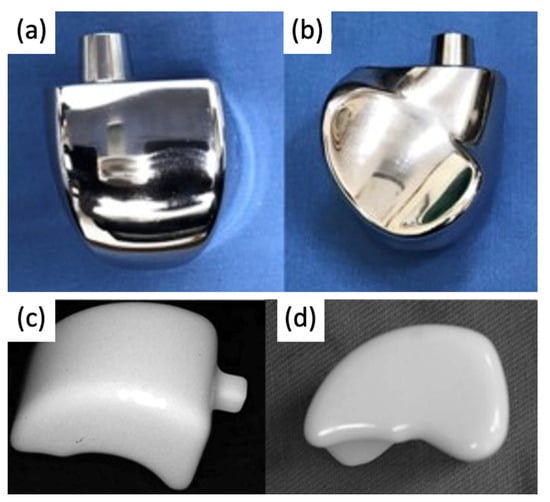
2.2. Current Total Ankle Arthroplasty Implants
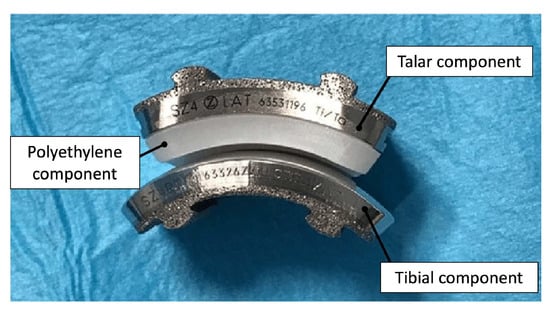
2.3. Advancements in Total Ankle Arthroplasty Design and Manufacturing
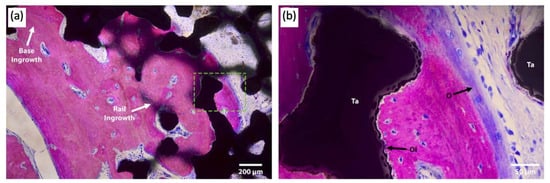
3. Total Talus Replacement
3.1. The Evolution of Total Talus Arthroplasty Implants
3.2. Current Total Talus Arthroplasty Implants
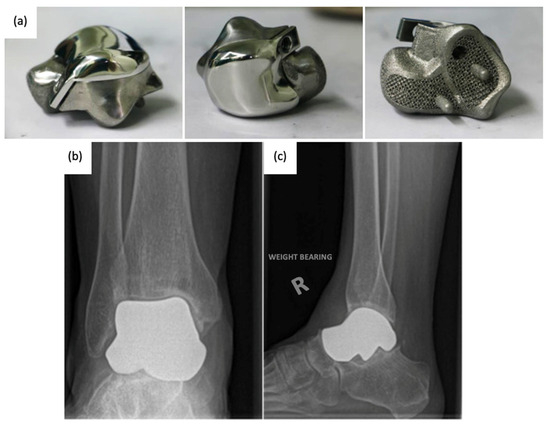
3.3. Advancements in Total Talus Arthroplasty Design and Manufacturing
4. Ankle Prosthetics
4.1. Evolution of Ankle Prosthetics
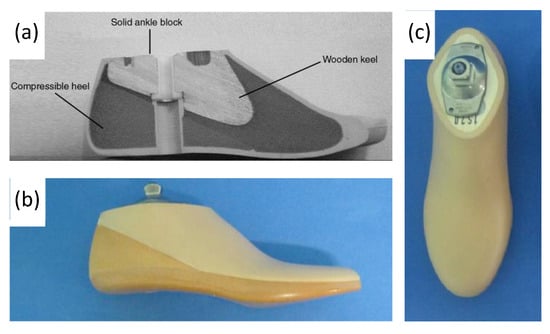
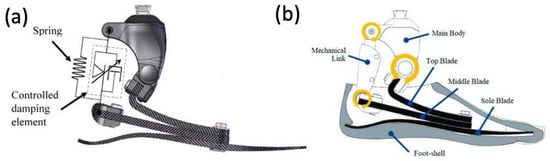
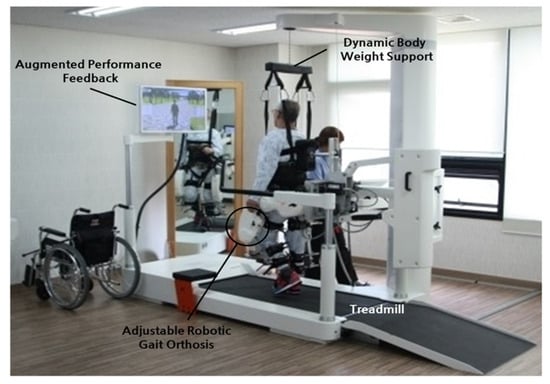
4.2. Early Powered Exoskeletons in Gait Rehabilitation
4.3. Battery-Powered Exoskeletons in Congenital Disorder Gait Rehabilitation
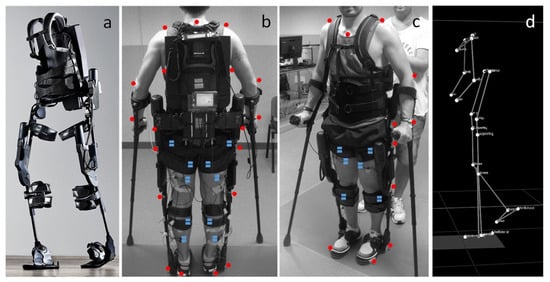
4.4. Powered Exoskeletons in Stroke Rehabilitation
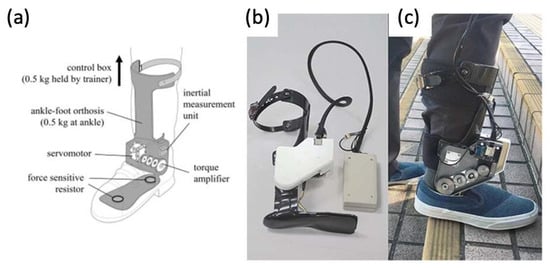
4.5. Powered Exoskeletons in Lower Extremity Trauma Patients
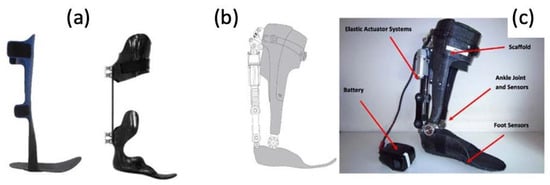
This entry is adapted from the peer-reviewed paper 10.3390/mi14112081
References
- Hansen, R.; Shibuya, N.; Jupiter, D.C. An Updated Epidemiology of Foot and Ankle Fractures in the United States: Complications, Mechanisms, and Risk Factors. J. Foot Ankle Surg. 2022, 61, 1034–1038.
- Bielska, I.A.; Wang, X.; Lee, R.; Johnson, A.P. The Health Economics of Ankle and Foot Sprains and Fractures: A Systematic Review of English-Language Published Papers. Part 2: The Direct and Indirect Costs of Injury. Foot 2019, 39, 115–121.
- Beckenkamp, P.R.; Lin, C.-W.C.; Chagpar, S.; Herbert, R.D.; van der Ploeg, H.P.; Moseley, A.M. Prognosis of Physical Function Following Ankle Fracture: A Systematic Review with Meta-Analysis. J. Orthop. Sports Phys. Ther. 2014, 44, 841–851.
- Pitkin, M.R. Effects of Design Variants in Lower-Limb Prostheses on Gait Synergy. J. Prosthet. Orthot. 1997, 9, 113–122.
- Wang, C.; Sun, B.; Zhang, Y.; Wang, C.; Yang, G. Design of a Novel Trabecular Acetabular Cup and Selective Laser Melting Fabrication. Materials 2022, 15, 6142.
- Ankle Replacement Surgery: How It Works, Recovery Time | HSS. Available online: https://www.hss.edu/condition-list_ankle-replacement-arthroplasty.asp (accessed on 12 April 2023).
- Arthritis of the Foot and Ankle—OrthoInfo—AAOS. Available online: https://orthoinfo.aaos.org/en/diseases--conditions/arthritis-of-the-foot-and-ankle/ (accessed on 12 April 2023).
- Noori, N.B.; Ouyang, J.Y.; Noori, M.; Altabey, W.A. A Review Study on Total Ankle Replacement. Appl. Sci. 2023, 13, 535.
- Ankle Fusion. Available online: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/ankle-fusion (accessed on 12 April 2023).
- Fuchs, S.; Sandmann, C.; Skwara, A.; Chylarecki, C. Quality of Life 20 Years after Arthrodesis of the Ankle. A Study of Adjacent Joints. J. Bone Jt. Surg. Br. 2003, 85, 994–998.
- Vickerstaff, J.A.; Miles, A.W.; Cunningham, J.L. A Brief History of Total Ankle Replacement and a Review of the Current Status. Med. Eng. Phys. 2007, 29, 1056–1064.
- Bonasia, D.E.; Dettoni, F.; Femino, J.E.; Phisitkul, P.; Germano, M.; Amendola, A. Total Ankle Replacement: Why, When and How? Iowa Orthop. J. 2010, 30, 119–130.
- Van Heiningen, J.; Vliet Vlieland, T.P.; van der Heide, H.J. The Mid-Term Outcome of Total Ankle Arthroplasty and Ankle Fusion in Rheumatoid Arthritis: A Systematic Review. BMC Musculoskelet. Disord. 2013, 26, 306.
- Alsayel, F.; Alttahir, M.; Mosca, M.; Barg, A.; Herrera-Pérez, M.; Valderrabano, V. Mobile Anatomical Total Ankle Arthroplasty—Improvement of Talus Recentralization. J. Clin. Med. 2021, 10, 554.
- Roberto, A.; Brandão, D.P.M. Current And Emerging Insights On Total Ankle Replacement. Podiatry Today 2018, 31.
- DiDomenico, L.A.; Anania, M.C. Total Ankle Replacements: An Overview. Clin. Podiatr. Med. Surg. 2011, 28, 727–744.
- Lawton, C.D.; Butler, B.A.; Dekker, R.G.; Prescott, A.; Kadakia, A.R. Total Ankle Arthroplasty versus Ankle Arthrodesis—A Comparison of Outcomes over the Last Decade. J. Orthop. Surg. Res. 2017, 12, 76.
- Martinelli, N.; Baretta, S.; Pagano, J.; Bianchi, A.; Villa, T.; Casaroli, G.; Galbusera, F. Contact Stresses, Pressure and Area in a Fixed-Bearing Total Ankle Replacement: A Finite Element Analysis. BMC Musculoskelet. Disord. 2017, 18, 493.
- Brigido, S.A.; DiDomenico, L.A. Primary Zimmer Trabecular Metal Total Ankle Replacement. In Primary and Revision Total Ankle Replacement; Roukis, T.S., Berlet, G.C., Bibbo, C., Hyer, C.F., Penner, M.J., Wünschel, M., Prissel, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 131–149. ISBN 978-3-319-24413-6.
- Epperson, R.T.; Barg, A.; Williams, D.L.; Saltzman, C.L. Histological Analysis of a Retrieved Porous Tantalum Total Ankle Replacement: A Case Report. JBJS Case Connect. 2020, 10, e0379.
- Talus Fractures—OrthoInfo—AAOS. Available online: https://www.orthoinfo.org/en/diseases--conditions/talus-fractures (accessed on 12 April 2023).
- Harnroongroj, T.; Tharmviboonsri, T.; Chuckpaiwong, B.; Chuckpaiwong, B.; Harnroongroj, T. The talar body prosthesis treated end-stage ankle arthritis with talar body deficient: A 6–13 years of follow-up outcomes and 6-year survivorship. Arch. Orthop. Trauma Surg. 2022, 142, 3083–3091.
- Harnroongroj, T.; Harnroongroj, T. The Talar Body Prosthesis: Results at Ten to Thirty-Six Years of Follow-Up. J. Bone Jt. Surg. Am. 2014, 96, 1211–1218.
- Taniguchi, A.; Takakura, Y.; Sugimoto, K.; Hayashi, K.; Ouchi, K.; Kumai, T.; Tanaka, Y. The Use of a Ceramic Talar Body Prosthesis in Patients with Aseptic Necrosis of the Talus. J. Bone Jt. Surg. Br. 2012, 94, 1529–1533.
- Tsukamoto, S.; Tanaka, Y.; Maegawa, N.; Shinohara, Y.; Taniguchi, A.; Kumai, T.; Takakura, Y. Total Talar Replacement Following Collapse of the Talar Body as a Complication of Total Ankle Arthroplasty: A Case Report. J. Bone Jt. Surg. Am. 2010, 92, 2115–2120.
- Gadkari, K.P.; Anderson, J.G.; Bohay, D.R.; Maskill, J.D.; Padley, M.A.; Behrend, L.A. An Eleven-Year Follow-up of a Custom Talar Prosthesis After Open Talar Extrusion in an Adolescent Patient: A Case Report. JBJS Case Connect. 2013, 3, e118.
- Taniguchi, A.; Takakura, Y.; Tanaka, Y.; Kurokawa, H.; Tomiwa, K.; Matsuda, T.; Kumai, T.; Sugimoto, K. An Alumina Ceramic Total Talar Prosthesis for Osteonecrosis of the Talus. J. Bone Jt. Surg. Am. 2015, 97, 1348–1353.
- Ando, Y.; Yasui, T.; Isawa, K.; Tanaka, S.; Tanaka, Y.; Takakura, Y. Total Talar Replacement for Idiopathic Necrosis of the Talus: A Case Report. J. Foot Ankle Surg. 2016, 55, 1292–1296.
- Stevens, B.W.; Dolan, C.M.; Anderson, J.G.; Bukrey, C.D. Custom Talar Prosthesis After Open Talar Extrusion in a Pediatric Patient. Foot Ankle Int. 2007, 28, 933–938.
- Yang, Q.D.; Mu, M.D.; Tao, X.; Tang, K.L. Three-dimensional printed talar prosthesis with biological function for giant cell tumor of the talus: A case report and review of the literature. World J. Clin. Cases 2021, 9, 3147–3156.
- Ruatti, S.; Corbet, C.; Boudissa, M.; Kerschbaumer, G.; Milaire, M.; Merloz, P.; Tonetti, J. Total Talar Prosthesis Replacement after Talar Extrusion. J. Foot Ankle Surg. 2017, 56, 905–909.
- Tonogai, I.; Hamada, D.; Yamasaki, Y.; Wada, K.; Takasago, T.; Tsutsui, T.; Goto, T.; Sairyo, K. Custom-Made Alumina Ceramic Total Talar Prosthesis for Idiopathic Aseptic Necrosis of the Talus: Report of Two Cases. Case Rep. Orthop. 2017, 2017, 8290804.
- Bowes, J.; Adeeb, S.; Grosvenor, A.; Beaupre, L.; Jomha, N.M. Development and Implantation of a Universal Talar Prosthesis. Front. Surg. 2019, 6, 63.
- Gorgey, A.S. Robotic Exoskeletons: The Current Pros and Cons. World J. Orthop. 2018, 9, 112–119.
- Lathouwers, E.; Díaz, M.A.; Maricot, A.; Tassignon, B.; Cherelle, C.; Cherelle, P.; Meeusen, R.; De Pauw, K. Therapeutic Benefits of Lower Limb Prostheses: A Systematic Review. J. Neuroeng. Rehabil. 2023, 20, 4.
- Knight, A.D.; Bass, S.R.; Elrod, J.M.; Hassinger, L.M.; Dearth, C.L.; Gonzalez-Vargas, J.; Hendershot, B.D.; Han, Z. Toward Developing a Powered Ankle-Foot Prosthesis With Electromyographic Control to Enhance Functional Performance: A Case Study in a U.S. Service Member. Mil. Med. 2022, usac038, e2772–e2777.
- Adalarasu, K. Comparison on Jaipur, SACH and Madras Foot A Psychophysiological Study. Int. J. Adv. Eng. Sci. Technol. 2011, 4, 187–192.
- Rehab & Prosthetics—Basic Science—Orthobullets. Available online: https://www.orthobullets.com/basic-science/9072/rehab-and-prosthetics (accessed on 17 July 2023).
- Paradisi, F.; Delussu, A.S.; Brunelli, S.; Iosa, M.; Pellegrini, R.; Zenardi, D.; Traballesi, M. The Conventional Non-Articulated SACH or a Multiaxial Prosthetic Foot for Hypomobile Transtibial Amputees? A Clinical Comparison on Mobility, Balance, and Quality of Life. ScientificWorldJournal 2015, 2015, 261801.
- Fey, N.P.; Klute, G.K.; Neptune, R.R. Optimization of Prosthetic Foot Stiffness to Reduce Metabolic Cost and Intact Knee Loading During Below-Knee Amputee Walking: A Theoretical Study. J. Biomech. Eng. 2012, 134, 1110051–11100510.
- Tryggvason, H.; Starker, F.; Lecomte, C.; Jonsdottir, F. Use of Dynamic FEA for Design Modification and Energy Analysis of a Variable Stiffness Prosthetic Foot. Appl. Sci. 2020, 10, 650.
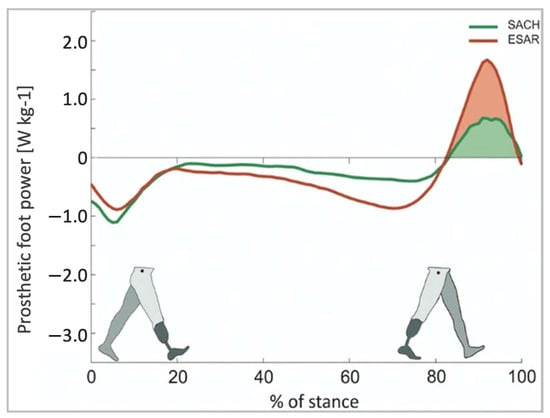
- Müller, R.; Tronicke, L.; Abel, R.; Lechler, K. Prosthetic Push-off Power in Trans-Tibial Amputee Level Ground Walking: A Systematic Review. PLoS ONE 2019, 14, e0225032.
- Houdijk, H.; Wezenberg, D.; Hak, L.; Cutti, A.G. Energy Storing and Return Prosthetic Feet Improve Step Length Symmetry While Preserving Margins of Stability in Persons with Transtibial Amputation. J. NeuroEng. Rehabil. 2018, 15, 76.
- Choi, W. Effects of Robot-Assisted Gait Training with Body Weight Support on Gait and Balance in Stroke Patients. Int. J. Environ. Res. Public Health 2022, 19, 5814.
- De Luca, A.; Bellitto, A.; Mandraccia, S.; Marchesi, G.; Pellegrino, L.; Coscia, M.; Leoncini, C.; Rossi, L.; Gamba, S.; Massone, A.; et al. Exoskeleton for Gait Rehabilitation: Effects of Assistance, Mechanical Structure, and Walking Aids on Muscle Activations. Appl. Sci. 2019, 9, 2868.
- Yeung, L.-F.; Lau, C.C.Y.; Lai, C.W.K.; Soo, Y.O.Y.; Chan, M.-L.; Tong, R.K.Y. Effects of Wearable Ankle Robotics for Stair and Over-Ground Training on Sub-Acute Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 19.
- Yeung, L.-F.; Ockenfeld, C.; Pang, M.-K.; Wai, H.-W.; Soo, O.-Y.; Li, S.-W.; Tong, K.-Y. Randomized Controlled Trial of Robot-Assisted Gait Training with Dorsiflexion Assistance on Chronic Stroke Patients Wearing Ankle-Foot-Orthosis. J. Neuroeng. Rehabil. 2018, 15, 51.
- Russell Esposito, E.; Schmidtbauer, K.A.; Wilken, J.M. Experimental Comparisons of Passive and Powered Ankle-Foot Orthoses in Individuals with Limb Reconstruction. J. Neuroeng. Rehabil. 2018, 15, 111.
