Mediastinal masses present a diagnostic challenge due to their diverse etiologies. Accurate localization and internal characteristics of the mass are the two most important factors to narrow the differential diagnosis or provide a specific diagnosis. The International Thymic Malignancy Interest Group (ITMIG) classification is the standard classification system used to localize mediastinal masses. Computed tomography (CT) and magnetic resonance imaging (MRI) are the two most commonly used imaging modalities for characterization of the mediastinal masses.
- mediastinum
- imaging
- CT
- MRI
1. Introduction
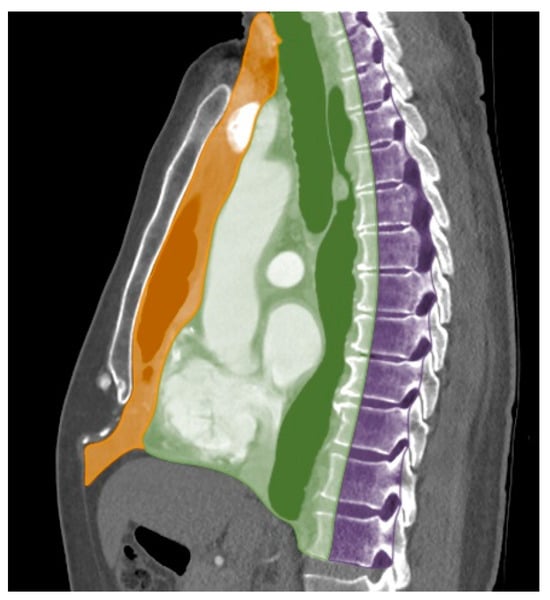
2. Imaging Modalities
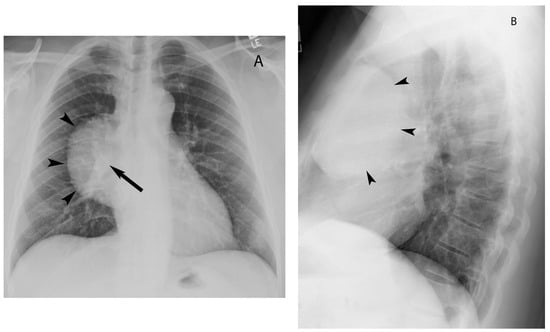
3. Cystic Lesions
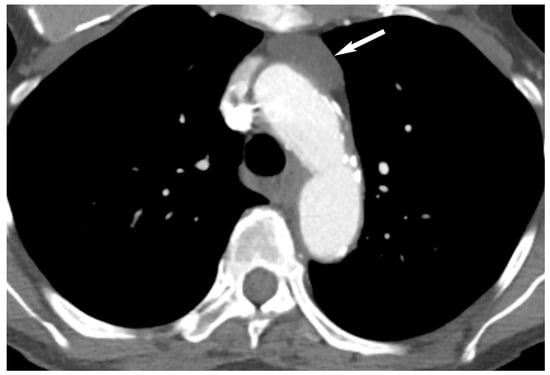
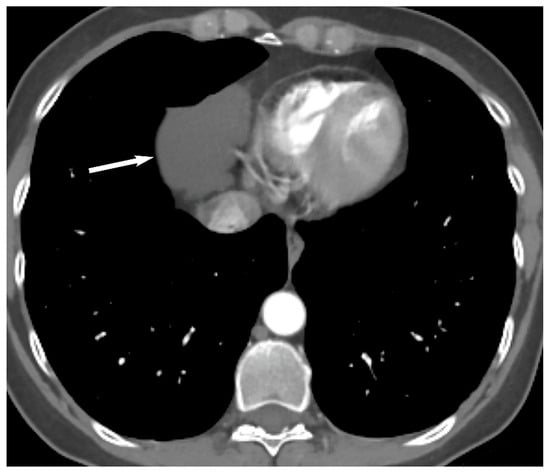
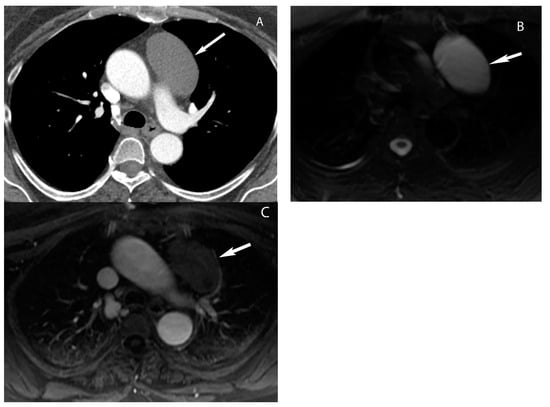
4. Thymic Hyperplasia
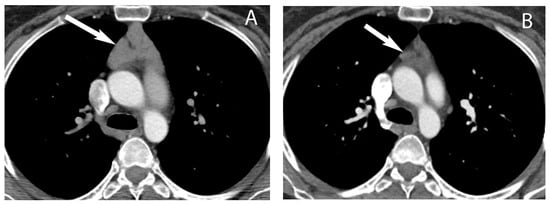
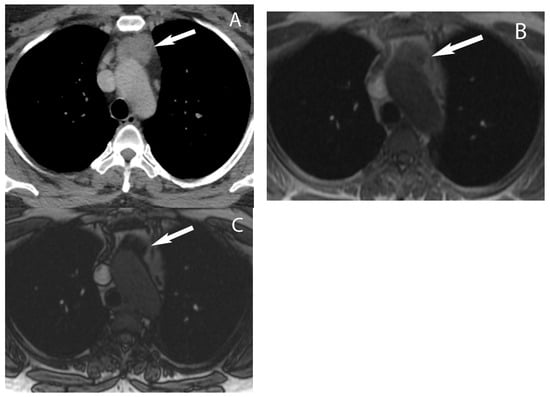
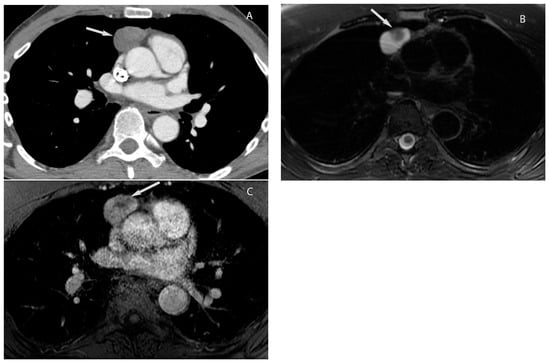
5. Thymic Epithelial Neoplasms
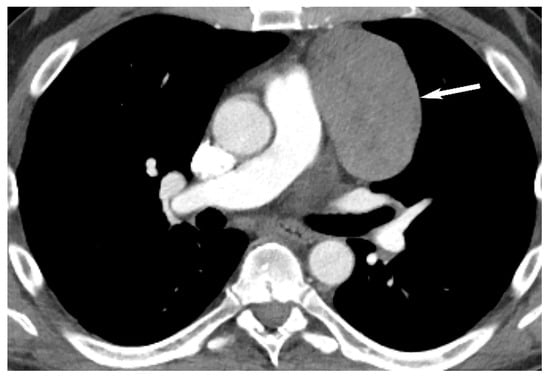
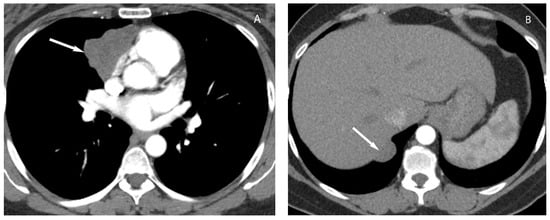
6. Lymphoma
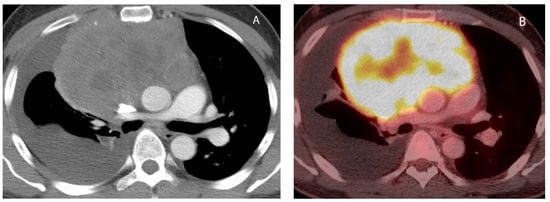
7. Germ Cell Tumors
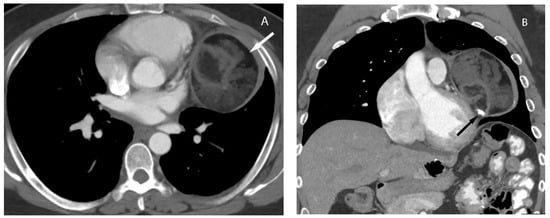
Upon CT, teratomas usually present as a well-defined heterogeneous mass with variable amounts of fat and soft tissue and may contain calcification (Figure 12). Rarely, bone or tooth can be identified within the mass. They may be predominantly cystic (unilocular or multilocular) and the presence of a fat fluid level within the mass is highly suggestive of mature teratoma but less commonly seen [4][18][29].
8. Cystic Lesions
The most common cystic lesions in the visceral compartment include bronchogenic and esophageal duplication cysts. Bronchogenic cyst commonly occurs near the carina or less frequently in the right paratracheal region. Upon CT, it typically manifests as well-circumscribed, homogeneous lesions with fluid attenuation (0 to 20 HU). The cyst wall may show enhancement with contrast administration or contain calcification. Internal heterogeneity can occur in some cases due to hemorrhagic or proteinaceous contents or infection of the bronchogenic cyst. MRI is helpful in such cases to confirm the cystic nature of these legions. MRI shows high signal intensity on T2-weighted images and variable signal intensity on T1-weighted images depending upon the hemorrhagic or proteinaceous contents of the cystic lesion. Typically, these lesions do not enhance but sometimes enhancement of the wall may be seen after gadolinium administration (Figure 13) [11][12][30].
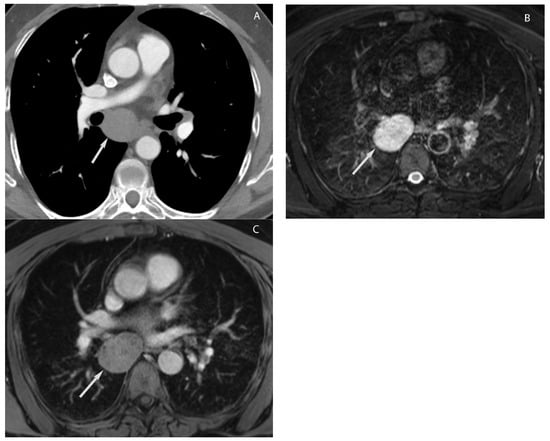
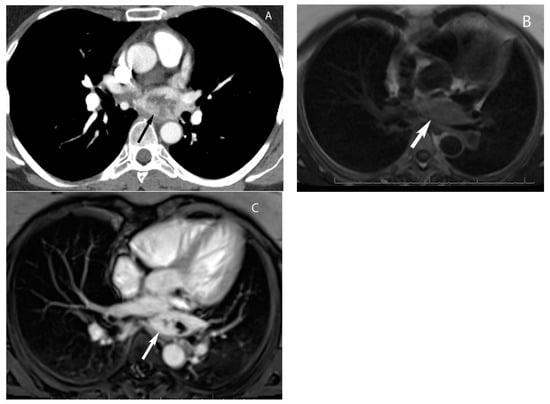
9. Hypervascular Lesions
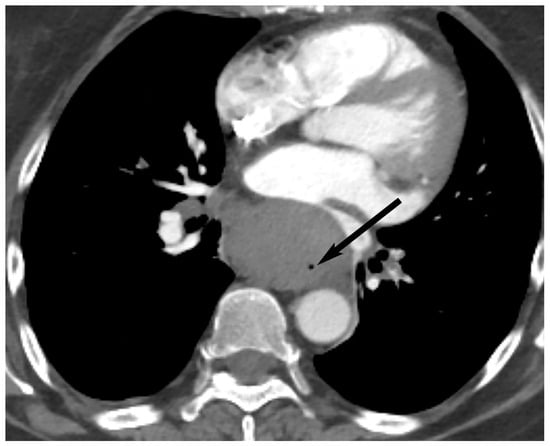
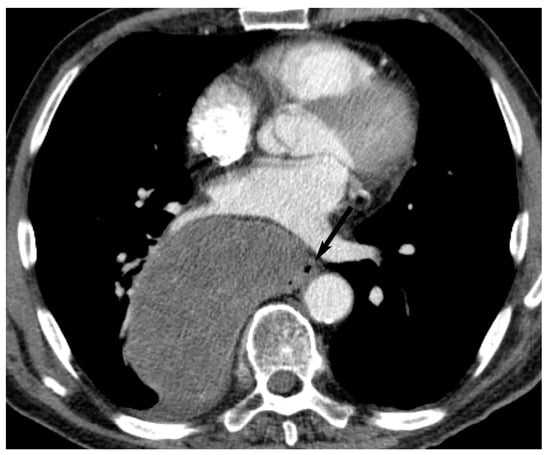
10. Esophageal Lesions
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13203171
References
- Carter, B.W.; Tomiyama, N.; Bhora, F.Y.; de Christenson, M.L.R.; Nakajima, J.; Boiselle, P.M.; Detterbeck, F.C.; Marom, E.M. A modern definition of mediastinal compartments. J. Thorac. Oncol. 2014, 9, S97–S101.
- Carter, B.W.; Benveniste, M.F.; Madan, R.; Godoy, M.C.; De Groot, P.M.; Truong, M.T.; Rosado-De-Christenson, M.L.; Marom, E.M. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017, 37, 413–436.
- Bhalla, S.; Marom, E. Approach to Imaging of Mediastinal Conditions in the Adult. In Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2019; pp. 27–35.
- Tomiyama, N. Approach to the prevascular mass. Mediastinum 2019, 3, 17.
- Verma, S.; Kalra, K.; Rastogi, S.; Sidhu, H.S. Clinical approach to childhood mediastinal tumors and management. Mediastinum 2020, 4, 21.
- D’Cruz, I.A.; Feghali, N.; Gross, C.M. Echocardiographic manifestations of mediastinal masses compressing or encroaching on the heart. Echocardiography 1994, 11, 523–533.
- Hovgaard, H.L.; Nielsen, R.R.; Laursen, C.B.; Frederiksen, C.A.; Juhl-Olsen, P. When appearances deceive: Echocardiographic changes due to common chest pathology. Echocardiography 2018, 35, 1847–1859.
- Garrana, S.H.; Rosado-de-Christenson, M.L. Imaging of the Anterior/Prevascular Mediastinum. Radiol. Clin. N. Am. 2021, 59, 155–168.
- Wang, X.; Chen, K.; Li, X.; Li, Y.; Yang, F.; Li, J.; Jiang, G.; Liu, J.; Wang, J. Clinical features, diagnosis and thoracoscopic surgical treatment of thymic cysts. J. Thorac. Dis. 2017, 9, 5203–5211.
- Shah, A.; Rojas, C.A. Imaging modalities (MRI, CT, PET/CT), indications, differential diagnosis and imaging characteristics of cystic mediastinal masses: A review. Mediastinum 2023, 7, 3.
- Carter, B.W.; Betancourt, S.L.; Benveniste, M.F. MR Imaging of Mediastinal Masses. Top. Magn. Reson. Imaging 2017, 26, 153–165.
- Ackman, J.B. MR Imaging of Mediastinal Masses. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 141–164.
- Oramas, D.M.; Moran, C.A. Multilocular thymic cyst (MTC) and other tumors with MTC features: Pitfalls in diagnosis. Semin. Diagn. Pathol. 2022, 39, 105–112.
- Carter, B.W.; Benveniste, M.F.; Truong, M.T.; Marom, E.M. State of the Art: MR Imaging of Thymoma. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 165–177.
- Park, J.W.; Jeong, W.G.; Lee, J.E.; Lee, H.-J.; Ki, S.Y.; Lee, B.C.; Kim, H.O.; Kim, S.K.; Heo, S.H.; Lim, H.S.; et al. Pictorial Review of Mediastinal Masses with an Emphasis on Magnetic Resonance Imaging. Korean J. Radiol. 2021, 22, 139–154.
- Kim, J.H.; Choe, J.; Kim, H.K.; Lee, H.Y. MRI-Based Stepwise Approach to Anterior Mediastinal Cystic Lesions for Diagnosis and Further Management. Korean J. Radiol. 2023, 24, 62–78.
- Carter, B.W.; Lichtenberger, J.P., 3rd; Benveniste, M.F. MR Imaging of Thymic Epithelial Neoplasms. Top. Magn. Reson. Imaging 2018, 27, 65–71.
- Nakazono, T.; Yamaguchi, K.; Egashira, R.; Mizuguchi, M.; Irie, H. Anterior mediastinal lesions: CT and MRI features and differential diagnosis. Jpn. J. Radiol. 2021, 39, 101–117.
- Gentili, F.; Pelini, V.; Lucii, G.; Luzzi, L.; Mazzei, F.G.; Fausto, A.; Volterrani, L.; Mazzei, M.A. Update in diagnostic imaging of the thymus and anterior mediastinal masses. Gland. Surg. 2019, 8, S188–S207.
- Guleria, P.; Jain, D. Thymic lesions of the paediatric age group: A comprehensive review of non-neoplastic and neoplastic etiologies. Mediastinum 2019, 3, 24.
- Lichtenberger, J.P., 3rd; Carter, B.W.; Fisher, D.A.; Parker, R.F.; Peterson, P.G. Thymic Epithelial Neoplasms: Radiologic-Pathologic Correlation. Radiol. Clin. N. Am. 2021, 59, 169–182.
- Benveniste, M.F.K.; Betancourt Cuellar, S.L.; Carter, B.W.; Strange, C.D.; Marom, E.M. Thymic Epithelial Neoplasms: Tumor-Node-Metastasis Staging. Radiol. Clin. N. Am. 2021, 59, 183–192.
- Taka, M.; Kobayashi, S.; Mizutomi, K.; Inoue, D.; Takamatsu, S.; Gabata, T.; Matsumoto, I.; Ikeda, H.; Kobayashi, T.; Minato, H.; et al. Diagnostic approach for mediastinal masses with radiopathological correlation. Eur. J. Radiol. 2023, 162, 110767.
- Pfau, D.; Smith, D.A.; Beck, R.; Gilani, K.A.; Gupta, A.; Caimi, P.; Ramaiya, N.H. Primary Mediastinal Large B-Cell Lymphoma: A Review for Radiologists. AJR Am. J. Roentgenol. 2019, 213, W194–W210.
- Mallick, S.; Jain, S.; Ramteke, P. Pediatric mediastinal lymphoma. Mediastinum 2020, 4, 22.
- Zeman, M.N.; Akin, E.A.; Merryman, R.W.; Jacene, H.A. Interim FDG-PET/CT for Response Assessment of Lymphoma. Semin. Nucl. Med. 2023, 53, 371–388.
- Zanoni, L.; Bezzi, D.; Nanni, C.; Paccagnella, A.; Farina, A.; Broccoli, A.; Casadei, B.; Zinzani, P.L.; Fanti, S. PET/CT in Non-Hodgkin Lymphoma: An Update. Semin. Nucl. Med. 2023, 53, 320–351.
- McCarten, K.M.; Nadel, H.R.; Shulkin, B.L.; Cho, S.Y. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr. Radiol. 2019, 49, 1545–1564.
- Patnaik, S.; Malempati, A.R.; Uppin, M.; Susarla, R. Rare mediastinal masses-imaging review. J. Cancer Res. Ther. 2021, 17, 13–21.
- Wahi, J.E.; Safdie, F.M. Esophageal duplication cysts: A clinical practice review. Mediastinum 2023, 7, 1.
- Bourgouin, P.P.; Madan, R. Imaging of the Middle and Visceral Mediastinum. Radiol. Clin. N. Am. 2021, 59, 193–204.
- Brown, M.L.; Zayas, G.E.; Abel, M.D.; Young, W.F., Jr.; Schaff, H.V. Mediastinal paragangliomas: The mayo clinic experience. Ann. Thorac. Surg. 2008, 86, 946–951.
- Balcombe, J.; Torigian, D.A.; Kim, W.; Miller, W.T., Jr. Cross-sectional imaging of paragangliomas of the aortic body and other thoracic branchiomeric paraganglia. AJR Am. J. Roentgenol. 2007, 188, 1054–1058.
- Lichtenberger, J.P., 3rd; Zeman, M.N.; Dulberger, A.R.; Alqutub, S.; Carter, B.W.; Manning, M.A. Esophageal Neoplasms: Radiologic-Pathologic Correlation. Radiol. Clin. N. Am. 2021, 59, 205–217.
- Cummings, D.; Wong, J.; Palm, R.; Hoffe, S.; Almhanna, K.; Vignesh, S. Epidemiology, Diagnosis, Staging and Multimodal Therapy of Esophageal and Gastric Tumors. Cancers 2021, 13, 582.
