Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Hydatid cysts have been widely recognized for decades as a common medical problem that affects millions of people. A revolution in medical treatment may be on the prospect of nanotechnology enhancing chemotherapy against hydatid cysts.
- chemotherapy
- hydatid cyst
- nanotechnology
1. Introduction
Hydatid cyst disease is an important threat to people’s well-being [1]. This parasite can live on various hosts in various parts of the world, mainly pastoral and rural [2][3]. As a result of this parasitic disease, fluid-filled cysts develop, which can become substantial in size and silently destroy the host’s tissues and structures [4]. In humans and animals, silent progression can lead to a delay in diagnosis and limited treatment options, thus exacerbating the condition when it goes undiagnosed [5].
The fibrous layer of a hydatid cyst is derived from the host’s connective tissue, forming a protective barrier against the host’s immune response [6]. This is followed by a laminated layer of chitinous fibers arranged in precise laminations, which may contribute to maintaining mechanical stability and evading immune detection [7][8][9]. The innermost germinal layer produces protoscolices, an infective agent that facilitates attachment and further infection [10][11]. A parasite’s behavior in this layered structure reveals that it can adapt to its host’s environment to survive and spread on it [12].
In recent decades, research innovations and technological advances have merged to give rise to a new field of research known as nanotechnology, in which system components are engineered and designed at the nanoscale, a measurement requiring extreme accuracy and measured in billionths of meters [13][14]. This field can transcend conventional boundaries, allowing scientists to manipulate matter fundamentally [15]. The advent of nanotechnology promises to revolutionize medicine in the next few decades by opening up a whole new world of possibilities that could drastically change treatment methods [16][17]. The potential of nanotechnology to improve chemotherapy is one of the most promising aspects of its influence in the medical field [18][19]. The traditional method of chemotherapy involves administering therapeutic agents indifferently to both diseased and healthy tissues, resulting in harmful adverse effects during treatment [20][21]. The advent of nanotechnology promises to transcend this limitation by enabling these therapeutic agents to be controlled with unprecedented precision [22][23][24]. Through nanoparticles, chemotherapy drugs can be delivered to the target area with high accuracy to avoid collateral tissue injuries [25][26][27]. With a targeted delivery method, it is expected that the performance of the chemotherapy will be increased [28][29][30]. The side effects of conventional chemotherapy will be reduced, which often cause significant health problems [31][32][33]. Nanotechnology offers a promising avenue of innovation for treating hydatid cyst disease, which poses a formidable challenge to medical professionals [34][35][36]. Researchers have been capable of using nanoparticles to design treatment strategies that target cysts precisely with unique properties while minimizing adverse effects on healthy tissues due to their use [37][38][39]. With the potential to change the therapeutic landscape, this precise and efficient drug delivery system may become a saving grace for those suffering from this parasitic condition [40][41][42][43]. Hydatid cyst disease and nanotechnology synergize to create a compelling story of hope in the face of adversity [44][45][46][47].
Cyst ruptures and anaphylactic shock can be prevented by accurately diagnosing hydatid cysts. Although the diagnosis can be challenging because the condition is similar to others and reliable imaging techniques are required, several factors contribute to the challenge [48][49]. Detecting cysts in specific locations can also be problematic, adding to the diagnostic difficulties [50][51][52]. As a result, it is imperative to overcome these challenges to ensure that the treatment can occur effectively and on time. Nanobiosensors are superior tools for detecting hydatid cysts [53]. They not only offer exceptional sensitivity and versatility when it comes to detecting specific biomarkers, but they also improve diagnostic accuracy [54]. A further advantage of these nanoscale devices is that they enable rapid and non-invasive detection, which can be especially helpful for early-stage cysts, which conventional treatments would otherwise miss [55]. Through the integration of nanobiosensors with imaging technology, this technology has the potential to revolutionize hydatid cyst diagnosis [56].
2. Detection of Hydatid Cysts: Current Approaches and Limitations
To treat hydatid cysts effectively, it is crucial to detect them as soon as possible [57][58]. Unfortunately, it is still difficult to detect this condition promptly and reliably, given its deceptive nature and existing diagnostic limitations. However, imaging techniques, such as ultrasound, are useful for providing initial insights, but can often be misinterpreted because hydatid cysts can often be confused with other cystic lesions [59][60]. High-resolution imaging can be obtained with Computerized Tomography (CT) scans and Magnetic Resonance Imaging (MRI), though these methods are expensive and are not suitable for distinguishing cyst types [61][62][63].
Specific antibodies against E. granulosus antigens can be screened via serological tests, such as ELISA and Western Blotting, but these tests are also limited [64]. Unlike ELISA, the Western Blot is more specific [65][66]. However, it requires specialized equipment and proficiency, increasing the chance of false positives. The resemblance between hydatid cysts and other cystic lesions can complicate the diagnosis [67]. In addition, the current screening system often detects cysts after they have already grown considerably, thus preventing early intervention that might result in better outcomes [68].
Performing an aspiration and biopsy is often necessary to reach a definitive diagnosis [69]. However, these procedures are not without risk, including the possibility of rupture and cyst spreading. Sometimes, even serological tests can fail, especially when dealing with inactive cysts or cysts that have calcified over the years [70][71]. Further complicating cancer detection is the lack of advanced imaging and diagnostic technologies in areas with a low level of technology [72]. More research is needed on hydatid cysts, and more accurate and accessible diagnostic methods are required (Figure 1).
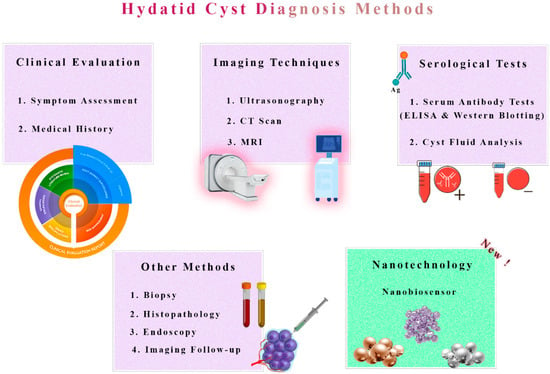
Figure 1. Traditional Diagnosis vs. Nanobiosensor Novelty in Hydatid Cyst Detection. A comparative representation highlights the contrast between traditional diagnostic methods and the innovative use of nanobiosensors in detecting hydatid cysts. Traditional techniques, such as imaging and serological tests, are depicted alongside the emerging nanobiosensor technology, which offers enhanced sensitivity and specificity for accurate and rapid diagnosis. This illustration underscores the potential revolution in hydatid cyst detection by nanobiosensors, promising improved early diagnosis and patient care.
3. Nanotechnology and Nanoparticle Applications
Regarding biomedical progress, nanotechnology is one of the most influential breakthroughs when it is seamlessly integrated with the multifaceted scope of veterinary medicine, particularly in the specialized and complex discipline of parasitology [73]. It is no wonder that the medical field has experienced groundbreaking advances due to nanotechnology in recent years since it measures in nanometers, on a very small scale [74]. Nanotechnology offers a spectacular array of possibilities to veterinary medicine, where parasitic infections still pose significant challenges [75]. Various materials have the ability to handle the pervasive challenges of parasitic infections, each possessing distinct characteristics that contribute to their effectiveness when it comes to combating parasitic infections [76].
Metal nanoparticles, such as gold and silver nanoparticles, are well known for their outstanding stability and ability to be easily functionalized [77][78]. Parasitology organizations are becoming more adept at delivering targeted drugs to parasites [79][80]. They can attach ligands that target specific parasites or host cell receptors in addition to their surface properties. Therefore, the absorption of medications and the effectiveness of treatment is enhanced [81].
This polymeric nanoparticle made of Poly(Lactic-co-Glycolic Acid) (PLGA) and chitosan is an excellent example of a biocompatible and controlled delivery of drugs that can be used in countless applications [82][83]. Their unique contribution involves the ability to house anthelmintic drugs and release them in a controlled manner, which ensures that medicinal agents remain compelling long enough in the body to maximize therapeutic effects [84]. This sophisticated process results in a reduction in the dosing frequency and reduced side effects, achieving superior clinical outcomes [85].
Liposomes are lipid-based nanoparticles that possess malleability and biocompatibility among their most notable properties [86][87]. A versatile platform for the delivery of parasitology drugs can be developed by using these lipid ensembles [88]. Hydrophilic and hydrophobic therapeutic compounds can be encapsulated in various ways, allowing for the development of comprehensive and efficient methods for managing parasitic diseases [88].
Nanocapsules are one of several nanocarriers among the wide variety of nanocarriers that serve various functions. There is a protective layer surrounding lipid or polymer capsules that contain therapeutic agents [88][89]. Utilizing this type of clever design guarantees the stability of encapsulated drugs, as well as targeted drug release, resulting in exact drug distribution right to the site of the parasite infection, providing maximum effectiveness [90]. A delicate balance must be struck to increase treatment efficacy while reducing side effects. A powerful composition for parasitic disease management is the result. Diagnostics and treatment for veterinary parasites are set to undergo a revolution as nanotechnology and veterinary parasitology merge [91][92]. Soon, as a result of recent developments in these fields, veterinary science and nanotechnology are predicted to continue to flourish, innovate, and, ultimately, redefine our approach to treating parasitic infections in companion animals (Figure 2).
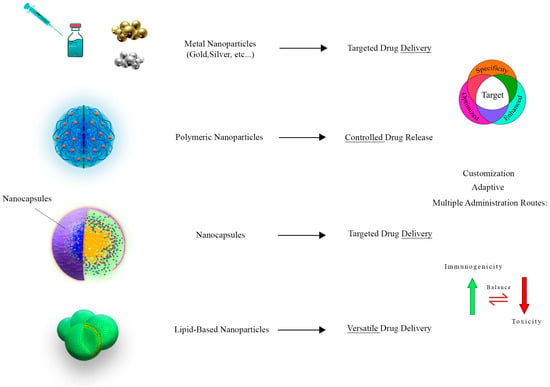
Figure 2. This figure shows the diverse applications of nanocarriers in the realm of parasitology. Three distinct categories of nanocarriers are featured, including metallic, polymeric, and lipid-based nanoparticles. Metallic nanoparticles, vividly exemplified by gold and silver nanoparticles, demonstrate their proficiency in targeted drug delivery, elevating treatment effectiveness by facilitating drug uptake. Polymeric nanoparticles, constructed from biocompatible materials like PLGA and chitosan, excel in controlled and sustained drug release, ultimately reducing dosing frequency and minimizing side effects. Meanwhile, lipid-based nanoparticles emerge as adaptable drug delivery platforms, adept at encapsulating hydrophilic drugs for comprehensive therapeutic approaches.
4. Revolutionizing Chemotherapy through Nanotechnology
In the past, conventional chemotherapy has been controversial because it is non-specific, often causing adverse effects on healthy tissues and resulting in limited therapeutic effectiveness [93][94]. A new era is now underway with the advent of nanotechnology and targeted drug delivery, promising many great treatment options [95][96]. It is possible to deliver therapeutic agents precisely at disease sites through nanoparticles, which are meticulously engineered with precise dimensions and functional groups [97]. Regarding hydatid cysts, nanoparticles can be tailored to target cysts while bypassing healthy tissues [98]. A nanoparticle can harmlessly pass through the intricate barriers within the host’s body [99].
4.1. Metallic Nanoparticles
Various nanomaterials are used to encapsulate praziquantel, such as gold, silver, zinc, copper, and zinc oxide, all composed of a combination of metals, to deliver the drug through nano-based delivery systems [100][101][102]. Materials like these function as carriers and offer intrinsic antimicrobial properties that contribute to the therapeutic effect.
According to Napooni et al. (2019), AuNPs display noteworthy protoscolicidal abilities [103]. These gold nanoparticles can be used as an alternative treatment for cystic echinococcosis because they eliminate the side effects of chemical drugs commonly used to treat this disease. These drugs are also effective when combined with solid lipid nanoparticles that contain albendazole and praziquantel. Based on a mouse model, Jelowdar et al. (2017) found that CE’s chemoprophylaxis outperformed free albendazole and Praziquantel [104]. It is, therefore, possible to conduct further studies by conducting clinical trials with this substance.
By synthesizing silver nanoparticles derived from Penicillium aculeatum, Rahimi et al. (2015) displayed protoscolicidal effects against E. granulosus [105]. The findings showed that Ag nanoparticles at all concentrations were remarkable protoscolicidal agents. Considering their biodegradability and harmless nature, these investigators believe AgNPs could be used as protoscolicidal agents.
Ag nanoparticles may help reduce the toxic effects of albendazole, the most commonly prescribed drug for treating hydatid disease. Aside from necrosis, degeneration, and steatosis, albendazole causes elevated serum hepatic enzyme levels. Nassef et al. (2019) found that encapsulating ABZ within Ag NPs makes it more potent at eliminating cystic echinococcosis [20].
It is well documented that Ag nanoparticles, amphotericin B, hypertonic saline, and Foeniculum vulgare essential oil have all been demonstrated to have protoscolicidal activity by Lashkarizadeh et al. (2015) [106]. The antiparasitic activity of AgNPs has been confirmed. Protoscoleces had a maximum activity level of 4 mg/mL, and 71.6% of them had died after an hour of exposure to it.
4.2. Antiparasitic-Loaded Nanodrugs
Nanostructured delivery methods are becoming increasingly popular to enhance the therapeutic effect of pharmaceutical agents and improve their bioavailability [107]. There is potential for a significant improvement in clinical efficacy and bioavailability due to a revolution in medical technology [108]. Nano-based formulations of albendazole and mebendazole, two widely used anthelmintic drugs, have been found to have promising results in treating hydatid cyst disease [109][110][111].
By applying ABZ sulfoxide-loaded chitosan-PGLA NPs produced by nanoprecipitation, Darvishi et al. (2020) demonstrated their utility in vivo [35]. A comparison was conducted between hemodialysis-treated cysts and sham surgery-treated cysts, and it was found that hemodialysis showed impressive therapeutic effects. As a result, the authors concluded that the mixture of ABZ sulfoxide-loaded chitosan-PGLA nanoparticles can assist in managing hydatid cyst disease in mice. The effects of ZrO2 nanoparticles on E. granulosus protoscoleces have been examined in a similar setting by Ibrahim (2020) [112]. Upon 60 min of incubation, ZrO2 NPs at 1000, 2000, and 4000 g/mL exhibited a notable contribution to the death of parasites in vivo. The specific delivery of the medication at the cyst site makes it both more efficient and minimizes collateral damage, which is one of the main downsides of conventional chemotherapy. Conventional chemotherapy is less discomforting by targeting the drug directly at the cyst. A closer look at the obstacles that hinder nanotechnology’s implementation, particularly in managing hydatid cysts, is necessary to understand its promise as a therapeutic technique [113].
With nanotechnology available to assist with manufacturing drug carriers, researchers can accomplish several benefits, including controlled drug release, precisely directed drug delivery, and more excellent solubility, all of which circumvent the weaknesses of traditional drug administration methods [114][115][116]. Using albendazoles and mebendazoles nanoencapsulated is a promising method of benzimidazole anthelmintics [117]. The encapsulation of these drugs has been achieved using various nanostructured carriers, including liposomes, polymeric nanoparticles, and lipid nanoparticles [118]. This significantly improves drug stability, circulation, and sustained release. It is possible to protect albendazole and mebendazole from rapid degradation by nanoencapsulation. In addition, nanoencapsulation makes it more likely for albendazole and mebendazole to accumulate selectively at the site of action. Further, praziquantel can also be implemented into nanoformulations to combat hydatid cysts, which play a crucial role in treating helminthic infections.
There are several mechanisms by which NPs are associated with antiparasitic activity, including the induction of apoptosis [119][120][121]. In an in vitro study, Naseri et al. (2016) evaluated albendazole sulfoxide and albendazole sulfoxide-loaded poly (lactic-co-glycolic acid)-PEG against protoscoleces [122]. In terms of apoptosis, they showed that ABZs and ABZs-loaded PLGA-PEG induced the cell death of protocoleces with oligonucleosomal DNA fragmentation. As a method for assessing whether these ABZs induce apoptosis in protoscoleces, caspase-3 mRNA synthesis by the genome of E. granulosus was tested.
Soltani et al. (2017) have reported that solid lipid nanoparticles loaded with albendazole and albendazole sulfoxide showed superior physicochemical and controlled release properties, indicating that these particles can act as highly efficient drug delivery particles [19]. These findings suggest that such materials might be beneficial for treating cystic echinococcosis. The synergistic administration of praziquantel-loaded nanocarriers with albendazole and mebendazole nanoformulations may improve treatment outcomes and compromise the emergence of drug resistance in hydatid cysts (Figure 3 and Figure 4).
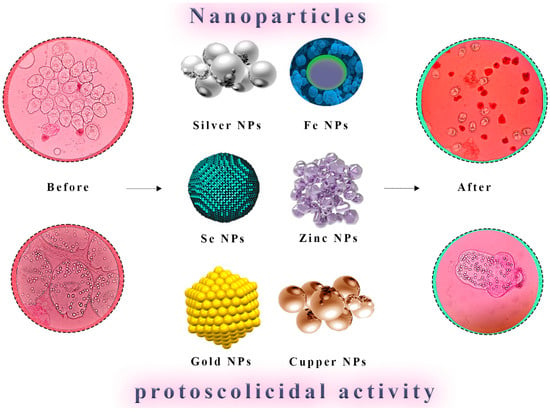
Figure 3. Direct Nanoparticle-Mediated Destruction of Hydatid Cyst Protoscoleces. A visual representation illustrates nanoparticles’ direct application for the targeted destruction of protoscoleces within hydatid cysts. Nanoparticles are shown interacting with protoscoleces, leading to their effective neutralization and elimination. This approach represents a promising avenue for the selective and efficient eradication of hydatid cysts.
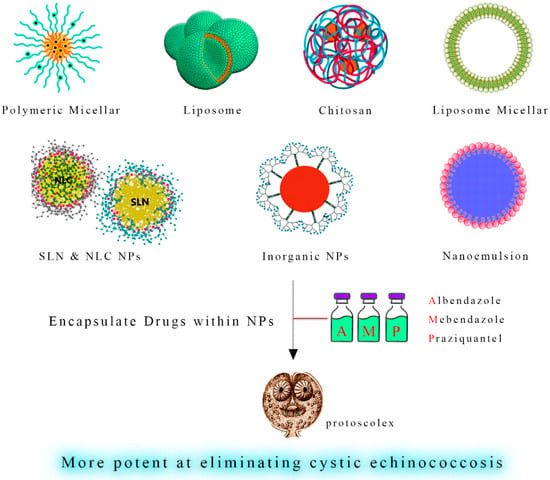
Figure 4. Enhanced Hydatid Cyst Treatment through Nano-Coatings. A schematic depiction showcases nano-coatings as a delivery platform for anthelmintic drugs such as albendazole, mebendazole, and praziquantel. These nano-coatings enable controlled and sustained release of the therapeutic agents, enhancing their action against hydatid cysts while minimizing systemic side effects. This innovative strategy can potentially optimize chemotherapy’s efficacy in managing hydatid cyst infections.
This entry is adapted from the peer-reviewed paper 10.3390/tropicalmed8110494
References
- Lupia, T.; Corcione, S.; Guerrera, F.; Costardi, L.; Ruffini, E.; Pinna, S.M.; Rosa, F.G.D. Pulmonary echinococcosis or lung hydatidosis: A narrative review. Surg. Infect. 2021, 22, 485–495.
- Gessese, A.T. Review on epidemiology and public health significance of hydatidosis. Vet. Med. Int. 2020, 2020, 8859116.
- Karshima, S.N.; Ahmed, M.I.; Adamu, N.B.; Magaji, A.A.; Zakariah, M.; Mohammed, K. Africa-wide meta-analysis on the prevalence and distribution of human cystic echinococcosis and canine Echinococcus granulosus infections. Parasit. Vectors 2022, 15, 357.
- Alvi, M.A.; Alsayeqh, A.F. Food-borne zoonotic echinococcosis: A review with special focus on epidemiology. Front. Vet. Sci. 2022, 9, 1072730.
- Casulli, A.; Massolo, A.; Saarma, U.; Umhang, G.; Santolamazza, F.; Santoro, A. Species and genotypes belonging to Echinococcus granulosussensu lato complex causing human cystic echinococcosis in Europe (2000–2021): A systematic review. Parasit. Vectors 2022, 15, 109.
- Sadr, S.; Charbgoo, A.; Borji, H.; Hajjafari, A. Interactions between innate immunity system and Echinococcus granulosus: Permission for vaccine development. Ser. Med. Sci. 2022, 3, 1–18.
- Díaz, Á. Immunology of cystic echinococcosis (hydatid disease). Br. Med. Bull. 2017, 124, 121–133.
- Hidalgo, C.; Stoore, C.; Strull, K.; Franco, C.; Corrêa, F.; Jiménez, M.; Hernández, M.; Lorenzatto, K.; Ferreira, H.B.; Galanti, N. New insights of the local immune response against both fertile and infertile hydatid cysts. PLoS ONE 2019, 14, e0211542.
- Díaz, A.; Casaravilla, C.; Irigoín, F.; Lin, G.; Previato, J.O.; Ferreira, F. Understanding the laminated layer of larval Echinococcus I: Structure. Trends. Parasitol. 2011, 27, 204–213.
- Goussard, P.; Eber, E.; Mfingwana, L.; Nel, P.; Schubert, P.; Janson, J.; Pitcher, R.; le Roux, C. Paediatric pulmonary echinococcosis: A neglected disease. Paediatr. Respir. Rev. 2022, 43, 11–23.
- Brehm, K.; Koziol, U. Echinococcus—Hst interactions at cellular and molecular levels. Adv. Parasitol. 2017, 95, 147–212.
- Thompson, R. Biology and systematics of Echinococcus. Adv. Parasitol. 2017, 95, 65–109.
- Sadr, S.; Poorjafari Jafroodi, P.; Haratizadeh, M.J.; Ghasemi, Z.; Borji, H.; Hajjafari, A. Current status of nano-vaccinology in veterinary medicine science. Vet. Med. Sci. 2023, 9, 2294–2308.
- Pramanik, P.K.D.; Solanki, A.; Debnath, A.; Nayyar, A.; El-Sappagh, S.; Kwak, K.-S. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access 2020, 8, 65230–65266.
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487.
- Sindhwani, S.; Chan, W.C. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498.
- Prasad, R.D.; Charmode, N.; Shrivastav, O.P.; Prasad, S.R.; Moghe, A.; Sarvalkar, P.D.; Prasad, N.R. A review on concept of nanotechnology in veterinary medicine. ES Food Agrofor. 2021, 4, 28–60.
- Shnawa, B.H.; Al-Ali, S.J.; Swar, S.O. Nanoparticles as a new approach for treating hydatid cyst disease. In Veterinary Pathobiology and Public Health; Unique Scientific Publishers: Punjab, Pakistan, 2021; pp. 180–189.
- Soltani, S.; Rafiei, A.; Ramezani, Z.; Abbaspour, M.R.; Jelowdar, A.; Kahvaz, M.S. Evaluation of the hydatid cyst membrane permeability of albendazole and albendazole sulfoxide-loaded solid lipid nanoparticles. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e34723.
- Nassef, N.E.; Saad, A.-G.E.; Harba, N.M.; Beshay, E.V.; Gouda, M.A.; Shendi, S.S.; Mohamed, A.S.E.-D. Evaluation of the therapeutic efficacy of albendazole-loaded silver nanoparticles against Echinococcus granulosus infection in experimental mice. J. Parasit. Dis. 2019, 43, 658–671.
- Fateh, R.; Norouzi, R.; Mirzaei, E.; Nissapatron, V.; Nawaz, M.; Khalifeh-Gholi, M.; Hamta, A.; Sadati, S.J.A.; Siyadatpanah, A.; Bafghi, A.F. In vitro evaluation of albendazole nanocrystals against Echinococcus granulosus protoscolices. Ann. Parasitol. 2021, 67, 203–212.
- Maurice, M.N.; Huseein, E.A.M.; Monib, M.E.S.M.; Alsharif, F.M.; Namazi, N.I.; Ahmad, A.A. Evaluation of the scolicidal activities of eugenol essential oil and its nanoemulsion against protoscoleces of hydatid cysts. PLoS ONE 2021, 16, e0259290.
- Alsharedeh, R.H.; Rezigue, M.; Bashatwah, R.M.; Amawi, H.; Aljabali, A.A.; Obeid, M.A.; Tambuwala, M.M. Nanomaterials as a Potential Target for Infectious Parasitic Agents. Curr. Drug Deliv. 2023, 250, 108548.
- Shnawa, B.H.; Jalil, P.J.; Aspoukeh, P.; Mohammed, D.A.; Biro, D.M. Protoscolicidal and Biocompatibility Properties of Biologically Fabricated Zinc Oxide Nanoparticles Using Ziziphus spina-christi Leaves. Pak. Vet. J. 2022, 42, 2074–7764.
- Rafiei, A.; Soltani, S.; Ramezani, Z.; Abbaspour, M.R.; Jelowdar, A.; Kahvaz, M.S. Ultrastructural changes on fertile and infertile hydatid cysts induced by conventional and solid lipid nanoparticles of albendazole and albendazole sulfoxide. Comp. Clin. Path. 2019, 28, 1045–1053.
- Çolak, B.; Aksoy, F.; Yavuz, S.; Demircili, M.E. Investigating the effect of gold nanoparticles on hydatid cyst protoscolices under low-power green laser irradiation. Turk. J. Surg. 2019, 35, 314.
- Aminpour, S.; Rafiei, A.; Jelowdar, A.; Kouchak, M. Evaluation of the protoscolicidal effects of albendazole and albendazole loaded solid lipid nanoparticles. Iran. J. Parasitol. 2019, 14, 127.
- Norouzi, R.; Ataei, A.; Hejazy, M.; Noreddin, A.; El Zowalaty, M.E. Scolicidal effects of nanoparticles against hydatid cyst protoscolices in vitro. Int. J. Nanomed. 2020, 2020, 1095–1100.
- Ahmadpour, E.; Godrati-Azar, Z.; Spotin, A.; Norouzi, R.; Hamishehkar, H.; Nami, S.; Heydarian, P.; Rajabi, S.; Mohammadi, M.; Perez-Cordon, G. Nanostructured lipid carriers of ivermectin as a novel drug delivery system in hydatidosis. Parasit. Vectors 2019, 12, 469.
- Taha, R.H. Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy. A review. Inorg. Chem. Commun. 2022, 143, 109610.
- Kohansal, K.; Rafiei, A.; Kalantari, H.; Jelowdar, A.; Salimi, A.; Rezaie, A.; Jalali, M.R. Nephrotoxicity of Albendazole and Albendazole Loaded Solid Lipid Nanoparticles in Mice with Experimental Hydatidosis. Adv. Pharm. Bull. 2022, 12, 102.
- Shnawa, B.H.; Hamad, S.M.; Barzinjy, A.A.; Kareem, P.A.; Ahmed, M.H. Scolicidal activity of biosynthesized zinc oxide nanoparticles by Mentha longifolia L. leaves against Echinococcus granulosus protoscolices. Emergent. Mater. 2021, 5, 683–693.
- Kishik, S.; Nagati, I.; El Hayawan, I.; Ali, I.; Fawzy, M.; Ali, H. Efficacy of Nigella sativa oil and its chitosan loaded nanoparticles on experimental cystic echinoncoccosis with immunological assessment. Parasitol. United J. 2020, 13, 172–178.
- Albalawi, A.E.; Alanazi, A.D.; Baharvand, P.; Sepahvand, M.; Mahmoudvand, H. High potency of organic and inorganic nanoparticles to treat cystic echinococcosis: An evidence-based review. Nanomaterials 2020, 10, 2538.
- Darvishi, M.M.; Moazeni, M.; Alizadeh, M.; Abedi, M.; Tamaddon, A.M. Evaluation of the efficacy of albendazole sulfoxide (ABZ-SO)–loaded chitosan-PLGA nanoparticles in the treatment of cystic echinococcosis in laboratory mice. Parasitol. Res. 2020, 119, 4233–4241.
- Hamad, S.M.; Shnawa, B.H.; Jalil, P.J.; Ahmed, M.H. Assessment of the therapeutic efficacy of silver nanoparticles against secondary cystic echinococcosis in BALB/c mice. Surfaces 2022, 5, 91–112.
- Salih, T.A.; Hassan, K.T.; Majeed, S.R.; Ibraheem, I.J.; Hassan, O.M.; Obaid, A. In vitro scolicidal activity of synthesised silver nanoparticles from aqueous plant extract against Echinococcus granulosus. Biotechnol. Rep. 2020, 28, e00545.
- Teimouri, A.; Jafarpour Azami, S.; Hashemi Hafshejani, S.; Ghanimatdan, M.; Bahreini, M.S.; Alimi, R.; Sadjjadi, S.M. Protoscolicidal effects of curcumin nanoemulsion against protoscoleces of Echinococcus granulosus. BMC Complement. Med. Ther. 2023, 23, 124.
- Navvabi, A.; Homaei, A.; Khademvatan, S.; Ansari, M.H.K.; Keshavarz, M. Combination of TiO2 nanoparticles and Echinometra mathaeis gonad extracts: In vitro and in vivo scolicidal activity against hydatid cysts. Biocatal. Agric. Biotechnol. 2019, 22, 101432.
- Firouzeh, N.; Eslaminejad, T.; Shafiei, R.; Faridi, A.; Fasihi Harandi, M. Lethal in vitro effects of optimized chitosan nanoparticles against protoscoleces of Echinococcus granulosus. J. Bioact. Compat. Polym. 2021, 36, 237–248.
- Jalil, P.J.; Shnawa, B.H.; Hamad, S.M. Silver Nanoparticles: Green Synthesis, Characterization, Blood Compatibility and Protoscolicidal Efficacy against Echinococcus granulosus. Pak. Vet. J. 2021, 41, 393–399.
- Norouzi, R.; Hejazy, M.; Ataei, A. Scolicidal activity of zinc oxide nanoparticles against hydatid cyst protoscolices in vitro. Nanomed. Res. J. 2019, 4, 23–28.
- Bakhtiar, N.M.; Akbarzadeh, A.; Ahmadpour, E.; Mahami-Oskouei, M.; Casulli, A.; Norouzi, R.; Asadi, M.; Ebrahimi, M.; Asadi, N.; Oliveira, S.M.R. In vitro efficacy of albendazole-loaded β-cyclodextrin against protoscoleces of Echinococcus granulosus sensu stricto. Exp. Parasitol. 2022, 243, 108428.
- Farhadi, M.; Haniloo, A.; Rostamizadeh, K.; Ahmadi, N. In vitro evaluation of albendazole-loaded nanostructured lipid carriers on Echinococcus granulosus microcysts and their prophylactic efficacy on experimental secondary hydatidosis. Parasitol. Res. 2021, 120, 4049–4060.
- Kishik, S.; Nagati, I.; Ali, I.; Aly, N.; Fawzy, M.; Ali, H. Pathological assessment of Nigella sativa oil and its chitosan loaded nanoparticles on experimental hepatic cystic echinoncoccosis. Parasitol. United J. 2021, 14, 58–62.
- Hamid, L.; Alsayari, A.; Tak, H.; Mir, S.A.; Almoyad, M.A.A.; Wahab, S.; Bader, G.N. An Insight into the Global Problem of Gastrointestinal Helminth Infections amongst Livestock: Does Nanotechnology Provide an Alternative? Agriculture 2023, 13, 1359.
- Liang, W.; Wang, X.-C.; Wu, X.-W.; Zhang, S.-J.; Sun, H.; Ma, X.; Peng, X.-Y. Efficacy of albendazole chitosan microspheres against Echinococcus granulosus infection in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2014, 32, 188–192.
- Saeedan, M.B.; Aljohani, I.M.; Alghofaily, K.A.; Loutfi, S.; Ghosh, S. Thoracic hydatid disease: A radiologic review of unusual cases. World. J. Clin. Cases 2020, 8, 1203.
- Barnes, T.S.; Deplazes, P.; Gottstein, B.; Jenkins, D.; Mathis, A.; Siles-Lucas, M.; Torgerson, P.R.; Ziadinov, I.; Heath, D.D. Challenges for diagnosis and control of cystic hydatid disease. Acta Trop. 2012, 123, 1–7.
- Rasheed, K.; Zargar, S.A.; Telwani, A.A. Hydatid cyst of spleen: A diagnostic challenge. N. Am. J. Med. Sci. 2013, 5, 10.
- Garg, M.K.; Sharma, M.; Gulati, A.; Gorsi, U.; Aggarwal, A.N.; Agarwal, R.; Khandelwal, N. Imaging in pulmonary hydatid cysts. World J. Radiol. 2016, 8, 581.
- Inayat, F.; Azam, S.; Baig, A.S.; Nawaz, G.; Ullah, W. Presentation patterns, diagnostic modalities, management strategies, and clinical outcomes in patients with hydatid disease of the pelvic bone: A comparative review of 31 cases. Cureus 2019, 11, e4178.
- Jafari, F.; Maghsood, A.H.; Fallah, M.; Jalilvand, A.; Matini, M.; Amini, B. Design of highly sensitive nano-biosensor for diagnosis of hydatid cyst based on gold nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 38, 102786.
- Jain, U.; Shakya, S.; Saxena, K. Nano-Biosensing Devices Detecting Biomarkers of Communicable and Non-communicable Diseases of Animals. In Biosensors in Agriculture: Recent Trends and Future Perspectives; Concepts and Strategies in Plant Sciences; Springer: Cham, Switzerland, 2021; pp. 415–434.
- Cerbu, C.; White, J.C.; Sabliov, C.M. Nanotechnology in livestock: Improving animal production and health. In Nano-Enabled Sustainable and Precision Agriculture; Elsevier: Amsterdam, The Netherlands, 2023; pp. 181–213.
- Hassan, A.A.; Sayed-ElAhl, R.M.; El Hamaky, A.M.; Mansour, M.K.; Oraby, N.H.; Barakat, M.H. Nanodiagnostics: New Tools for Detection of Animal Pathogens. In Nanorobotics and Nanodiagnostics in Integrative Biology and Biomedicine; Springer: Cham, Switzerland, 2022; pp. 299–325.
- Lightowlers, M.W.; Gasser, R.B.; Hemphill, A.; Romig, T.; Tamarozzi, F.; Deplazes, P.; Torgerson, P.R.; Garcia, H.H.; Kern, P. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int. J. Parasitol. 2021, 51, 1167–1192.
- Habibi, B.; Gholami, S.; Bagheri, A.; Fakhar, M.; Moradi, A.; Khazeei Tabari, M.A. Cystic echinococcosis microRnas as potential non-invasive biomarkers: Current insights and upcoming perspective. Expert Rev. Mol. Diagn. 2023, 23, 885–894.
- Dietrich, C.F.; Douira-Khomsi, W.; Gharbi, H.; Sharma, M.; Cui, X.W.; Sparchez, Z.; Richter, J.; Kabaalioğlu, A.; Atkinson, N.S.; Schreiber-Dietrich, D. Cystic and alveolar echinococcosis of the hepatobiliary tract—The role of new imaging techniques for improved diagnosis. Med. Ultrason. 2020, 22, 75–84.
- Noori, I.F.; Jabbar, A.S. Hepatic Hydatid Cyst Diseases during Pregnancy: Diagnosis, Management and Best Practice. Syst. Rev. Pharm. 2020, 11, 689–694.
- Caraiani, C.; Yi, D.; Petresc, B.; Dietrich, C. Indications for abdominal imaging: When and what to choose? J. Ultrason. 2020, 20, 43–54.
- Abbasi, B.; Akhavan, R.; Khameneh, A.G.; Amirkhiz, G.D.H.; Rezaei-Dalouei, H.; Tayebi, S.; Hashemi, J.; Aminizadeh, B.; Amirkhiz, S.D.H. Computed tomography and magnetic resonance imaging of hydatid disease: A pictorial review of uncommon imaging presentations. Heliyon 2021, 7, e07086.
- Calame, P.; Weck, M.; Busse-Cote, A.; Brumpt, E.; Richou, C.; Turco, C.; Doussot, A.; Bresson-Hadni, S.; Delabrousse, E. Role of the radiologist in the diagnosis and management of the two forms of hepatic echinococcosis. Insights Imaging 2022, 13, 68.
- Tamarozzi, F.; Silva, R.; Fittipaldo, V.A.; Buonfrate, D.; Gottstein, B.; Siles-Lucas, M. Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: A systematic review of the literature with meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009370.
- Alvi, M.A.; Ali, R.M.A.; Khan, S.; Saqib, M.; Qamar, W.; Li, L.; Fu, B.-Q.; Yan, H.-B.; Jia, W.-Z. Past and Present of Diagnosis of Echinococcosis: A Review (1999–2021). Acta Trop. 2023, 243, 106925.
- Meftahi, G.H.; Bahari, Z.; Zarei Mahmoudabadi, A.; Iman, M.; Jangravi, Z. Applications of western blot technique: From bench to bedside. Biochem. Mol. Biol. Educ. 2021, 49, 509–517.
- Alshoabi, S.A.; Alkalady, A.H.; Almas, K.M.; Magram, A.O.; Algaberi, A.K.; Alareqi, A.A.; Hamid, A.M.; Alhazmi, F.H.; Qurashi, A.A.; Abdulaal, O.M. Hydatid disease: A radiological pictorial review of a great neoplasms mimicker. Diagnostics 2023, 13, 1127.
- Chouhan, M.; Wiley, E.; Chiodini, P.; Amin, Z. Hepatic alveolar hydatid disease (Echinococcus multilocularis), a mimic of liver malignancy: A review for the radiologist in non-endemic areas. Clin. Radiol. 2019, 74, 247–256.
- Khalili, N.; Iranpour, P.; Khalili, N.; Haseli, S. Hydatid Disease: A Pictorial Review of Uncommon Locations. Iran. J. Med. Sci. 2023, 48, 118–129.
- Pakala, T.; Molina, M.; Wu, G.Y. Hepatic echinococcal cysts: A review. J. Clin. Transl. Hepatol. 2016, 4, 39.
- Paramita, A.A.K.Y.; Wibawa, I.D.N. Multimodal Treatment of Cystic Echinococcosis. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2023, 24, 72–82.
- Borhani, M.; Fathi, S.; Darabi, E.; Jalousian, F.; Simsek, S.; Ahmed, H.; Kesik, H.K.; Hosseini, S.H.; Romig, T.; Harandi, M.F. Echinococcoses in Iran, Turkey, and Pakistan: Old diseases in the new millennium. Clin. Microbiol. Rev. 2021, 34, e0029020.
- Fries, C.N.; Curvino, E.J.; Chen, J.-L.; Permar, S.R.; Fouda, G.G.; Collier, J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2021, 16, 1–14.
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered nanomaterials in soil: Their impact on soil microbiome and plant health. Plants 2021, 11, 109.
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Advances and challenges in nanocarriers and nanomedicines for veterinary application. Int. J. Pharm. 2020, 580, 119214.
- Sanabria, R. Nanotechnological improvement of veterinary anthelmintics. Pharm. Nanotechnol. 2021, 9, 5–14.
- Fatehbasharzad, P.; Fatehbasharzad, P.; Sillanpää, M.; Shamsi, Z. Investigation of bioimpacts of metallic and metallic oxide nanostructured materials: Size, shape, chemical composition, and surface functionality: A review. Part. Part. Syst. Charact. 2021, 38, 2100112.
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580.
- Hikal, W.M.; Bratovcic, A.; Baeshen, R.S.; Tkachenko, K.G.; Said-Al Ahl, H.A. Nanobiotechnology for the detection and control of waterborne parasites. Open J. Ecol. 2021, 11, 203–223.
- Badirzadeh, A.; Alipour, M.; Najm, M.; Vosoogh, A.; Vosoogh, M.; Samadian, H.; Hashemi, A.S.; Farsangi, Z.J.; Amini, S.M. Potential therapeutic effects of curcumin coated silver nanoparticle in the treatment of cutaneous leishmaniasis due to Leishmania major in-vitro and in a murine model. J. Drug Deliv. Sci. Technol. 2022, 74, 103576.
- Zhang, P.; Gong, J.; Jiang, Y.; Long, Y.; Lei, W.; Gao, X.; Guo, D. Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics 2023, 15, 1783.
- Ali, M.; Afzal, M.; Verma, M.; Bhattacharya, S.M.; Ahmad, F.; Samim, M.; Abidin, M.Z.; Dinda, A.K. Therapeutic efficacy of poly (lactic-co-glycolic acid) nanoparticles encapsulated ivermectin (nano-ivermectin) against brugian filariasis in experimental rodent model. Parasitol. Res. 2014, 113, 681–691.
- Wang, Q.; Sun, X.; Huang, X.; Huang, J.; Hasan, M.W.; Yan, R.; Xu, L.; Song, X.; Li, X. Nanoparticles of Chitosan/Poly (D, L-Lactide-Co-Glycolide) enhanced the immune responses of haemonchus contortus HCA59 antigen in model mice. Int. J. Nanomed. 2021, 16, 3125–3139.
- Li, J.; Yang, Y.; Han, X.; Li, J.; Tian, M.; Qi, W.; An, H.; Wu, C.; Zhang, Y.; Han, S.; et al. Oral Delivery of Anti-Parasitic Agent-Loaded PLGA Nanoparticles: Enhanced Liver Targeting and Improved Therapeutic Effect on Hepatic Alveolar Echinococcosis. Int. J. Nanomed. 2023, 2021, 3069–3085.
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric nanoparticles for antimicrobial therapies: An up-to-date overview. Polymers 2021, 13, 724.
- Cheraghipour, K.; Rouzbahani, A.K.; Fallahi, S.; Taherpour, F.; Moradifard, F.; Shakib, P.; Lashgarian, H.E.; Marzban, A. Recent Advances in Therapeutic Strategies against Hydatid Cysts using Nanomaterials: A Systematic Review. Lett. Drug Des. Discov. 2023, 20, 1185–1193.
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. NBM. 2018, 14, 2023–2050.
- Sasidharan, S.; Saudagar, P. Encapsulation and delivery of antiparasitic drugs: A review. Encapsul. Act. Mol. Deliv. Syst. 2020, 2020, 323–342.
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193.
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B 2020, 196, 111305.
- Cruz, A.A.; Molento, M.B. Nanotechnology: Meeting the future of Veterinary Parasitology Research. Pesqui. Vet. Bras. 2015, 35, 842–843.
- Qadeer, A.; Ullah, H.; Sohail, M.; Safi, S.Z.; Rahim, A.; Saleh, T.A.; Arbab, S.; Slama, P.; Horky, P. Potential application of nanotechnology in the treatment, diagnosis, and prevention of schistosomiasis. Front. Bioeng. Biotechnol. 2022, 10, 1013354.
- Haby, M.M.; Sosa Leon, L.A.; Luciañez, A.; Nicholls, R.S.; Reveiz, L.; Donadeu, M. Systematic review of the effectiveness of selected drugs for preventive chemotherapy for Taenia solium taeniasis. PLoS Negl. Trop. Dis. 2020, 14, e0007873.
- Hassan, N.M.; Ghazy, A.A. Advances in diagnosis and control of anthelmintic resistant gastrointestinal helminths infecting ruminants. J. Parasit. Dis. 2022, 46, 901–915.
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361.
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661.
- Wang, S.; Ma, Y.; Wang, W.; Dai, Y.; Sun, H.; Li, J.; Wang, S.; Li, F. Status and prospect of novel treatment options toward alveolar and cystic echinococcosis. Acta Trop. 2022, 226, 106252.
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246.
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B. 2022, 12, 3456–3474.
- Bajwa, H.U.R.; Khan, M.K.; Abbas, Z.; Riaz, R.; Rehman, T.; Abbas, R.Z.; Almutairi, M.M.; Alshammari, F.A.; Alraey, Y. Nanoparticles: Synthesis and their role as potential drug candidates for the treatment of parasitic diseases. Life 2022, 12, 750.
- Król, G.; Fortunka, K.; Majchrzak, M.; Piktel, E.; Paprocka, P.; Mańkowska, A.; Lesiak, A.; Karasiński, M.; Strzelecka, A.; Durnaś, B. Metallic Nanoparticles and Core-Shell Nanosystems in the Treatment, Diagnosis, and Prevention of Parasitic Diseases. Pathogens 2023, 12, 838.
- Boudier, A.; Le Faou, A. Nanoparticles and Other Nanostructures and the Control of Pathogens: From Bench to Vaccines. Int. J. Mol. Sci. 2023, 24, 9063.
- Napooni, S.; Arbabi, M.; Delavari, M.; Hooshyar, H.; Rasti, S. Lethal effects of gold nanoparticles on protoscolices of hydatid cyst: In vitro study. Comp. Clin. Pathol. 2019, 28, 143–150.
- Jelowdar, A.; Rafiei, A.; Abbaspour, M.R.; Rashidi, I.; Rahdar, M. Efficacy of combined albendazol and praziquntel and their loaded solid lipid nanoparticles components in chemoprophylaxis of experimental hydatidosis. Asian Pac. J. Trop. Biomed. 2017, 7, 549–554.
- Rahimi, M.T.; Ahmadpour, E.; Esboei, B.R.; Spotin, A.; Koshki, M.H.K.; Alizadeh, A.; Honary, S.; Barabadi, H.; Mohammadi, M.A. Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosus protoscolices. Int. J. Surg. 2015, 19, 128–133.
- Lashkarizadeh, M.R.; Asgaripour, K.; Dezaki, E.S.; Harandi, M.F. Comparison of scolicidal effects of amphotricin B, silver nanoparticles, and Foeniculum vulgare Mill on hydatid cysts protoscoleces. Iran. J. Parasitol. 2015, 10, 206.
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano-based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Abidi, H.; Ghaedi, M.; Rafiei, A.; Jelowdar, A.; Salimi, A.; Asfaram, A.; Ostovan, A. Magnetic solid lipid nanoparticles co-loaded with albendazole as an anti-parasitic drug: Sonochemical preparation, characterization, and in vitro drug release. J. Mol. Liq. 2018, 268, 11–18.
- Bhatia, M.; Kumar, S.; Kapoor, A.; Lohan, S. A Review on the Drug Delivery Strategies for Parasitic Infections: Scope and Assertion. Drug Deliv. 2022, 12, 109–121.
- Sinha, S.; Sehgal, R. Nano-targeted drug delivery for parasitic infections. In Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 395–424.
- Ibrahim, A.A.-J. Scolicidal activity of zirconium oxide (ZrO2) nanoparticles against protoscolices of hydatid cysts. Indian J. Forensic Med. Toxicol. 2020, 14, 469–472.
- Joshi, G.; Quadir, S.S.; Yadav, K.S. Road map to the treatment of neglected tropical diseases: Nanocarriers interventions. J. Control. Release 2021, 339, 51–74.
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691.
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307.
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191.
- Pandian, S.R.K.; Panneerselvam, T.; Pavadai, P.; Govindaraj, S.; Ravishankar, V.; Palanisamy, P.; Sampath, M.; Sankaranarayanan, M.; Kunjiappan, S. Nano based approach for the treatment of neglected tropical diseases. Front. Nanotechnol. 2021, 3, 665274.
- Shukla, R.; Mourya, A.; Handa, M.; Ujjwal, R.R. Role of nanomedicines in neglected tropical diseases. In Nanopharmaceutical Advanced Delivery Systems; Scrivener Publishing: Beverly, MA, USA, 2021; pp. 407–446.
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526.
- Khashan, K.S.; Sulaiman, G.M.; Hussain, S.A.; Marzoog, T.R.; Jabir, M.S. Synthesis, characterization and evaluation of anti-bacterial, anti-parasitic and anti-cancer activities of aluminum-doped zinc oxide nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3677–3693.
- Ezzatkhah, F.; Khalaf, A.K.; Mahmoudvand, H. Copper nanoparticles: Biosynthesis, characterization, and protoscolicidal effects alone and combined with albendazole against hydatid cyst protoscoleces. Biomed. Pharmacother. 2021, 136, 111257.
- Naseri, M.; Akbarzadeh, A.; Spotin, A.; Akbari, N.A.R.; Mahami-Oskouei, M.; Ahmadpour, E. Scolicidal and apoptotic activities of albendazole sulfoxide and albendazole sulfoxide-loaded PLGA-PEG as a novel nanopolymeric particle against Echinococcus granulosus protoscoleces. Parasitol. Res. 2016, 115, 4595–4603.
This entry is offline, you can click here to edit this entry!
