Pancreatic cancer incidence is increasing yearly. The reasons are not well known. Unfortunately, this is one of the least treatable cancers. Standard chemotherapy treatments show poor results, as do targeted treatments. The only real improvement in pancreatic cancer in the last twenty years occurred in the surgical field, where neoadjuvant therapy and very early surgery have achieved better overall survival. The only secret of arriving early to surgery is early diagnosis, and the missing element for early diagnosis is screening.
1. Introduction
Pancreatic cancer (PC) is becoming a public health problem because the number of cases is constantly increasing [
1], and treatment results are quite poor [
2,
3,
4]. Incidence is growing between 0.5 and 1% each year. In 1985, PC was the eighth most frequent cause of cancer mortality [
5]. Thirty years later, it was the fourth [
6], but it has been forecast that it will be in second place by 2030–2040 [
7,
8].
Most patients are diagnosed in a late stage when surgery is not feasible, or metastases are already present. Symptomatic patients are usually incurable [
20]. At the time of diagnosis in 80 to 85% of patients, an invasive pancreatic tumor of 4 cm or more in diameter is present. In most of these cases, there are overt or occult metastases as well [
21,
22].
Inoperable patients can be treated with chemotherapy and radiotherapy; however their response rate is low, and survival time is short. Targeted treatments, such as poly (adenosine diphosphate [ADB]-ribose) polymerase inhibitors, are limited to a small subset of patients with BRCA1/BRCA2 mutations.
2. Natural History of Pancreatic Cancer
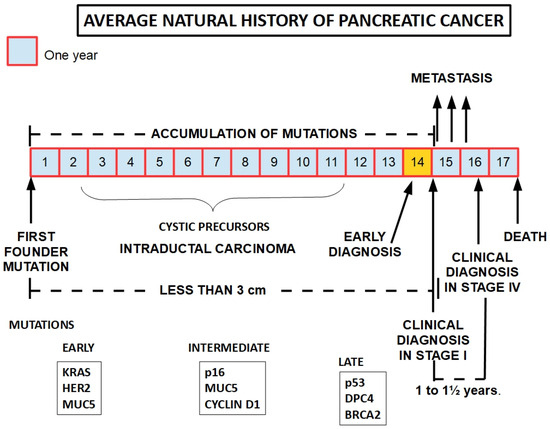
There is evidence that the symptomatic disease takes around fifteen years to develop from when the first pro-tumor genotypic change occurs [
29]. PDAC development follows a step-by-step process from intraepithelial dysplasia and intraductal neoplasia to full-blown invasive adenocarcinoma [
30,
31]. There is a series of precursor lesions, such as intraepithelial neoplasia and intraductal papillary mucinous tumors [
32] (the Sendai and Fukuoca protocols established when these cysts should be removed) [
33,
34].
Distant metastases also develop late [
26]. This evidence shows that contrary to what most textbooks say, PDAC is a slowly developing cancer. What probably creates the contradiction is that by the time it is diagnosed—usually late—the tumor has achieved cumulative mutations that accelerate its progression, and very frequently, distant metastases have already occurred. The time interval between early and late diagnosis is less than 1½ years (
Figure 1).
Intraepithelial neoplasms should be considered precancerous lesions. However, it is not clear which will progress to PDAC and which will remain unchanged [
35]. The KRAS mutation is insufficient for distinguishing those that will follow a malignant path because both can show this mutation [
36,
37].
On the other hand, the KRAS mutation is an important hallmark that helps rule out chronic pancreatitis [
38].
Figure 1. The development of pancreatic cancer is a slow process that takes around 15 years to become symptomatic. The pace of progression seems to accelerate concomitantly with the presentation of the first symptoms. In order to be therapeutically effective, early diagnosis needs to occur immediately before symptoms develop or very shortly afterwards. This therapeutic window may be as short as weeks or a few months at best. Lower panels show frequently found mutations according to the progression of the tumor [
39].
3. Growth of Pancreatic Cancer
The classic tool, although inaccurate, for measuring tumor growth is tumor volume duplication time (TVDT). TVDT is usually determined from two-volume estimations with measurement time intervals and establishes the time it takes to double the volume of a tumor. Unfortunately, there are many publications with very different results. Therefore, the information gathered in this regard should be considered with prudence.
Ahn et al. [
41] studied duplication time with CT scan in 110 patients with proven PDAC and found a very wide range that went from 20 to 977 days (mean 132 days): “
The growth rate was significantly associated with the initial diameter and volume. The development of distant metastasis was significantly associated with initial diameter, volume, and volume growth rate”.
4. A Growth Model of Pancreatic Cancer
Although not universally accepted, a growth model for pancreatic cancer progression can be built following the multistep scheme that Vogelstein, Feron, and Kinslay proposed for colorectal carcinoma [
43,
44].
In the pancreas, the initial lesion is probably a mutation of an intraductal cell, the founder mutation, leading to intraductal proliferation or intraductal hyperplasia [
45]. It is this dysplastic duct that evolves into the invasive adenocarcinoma through clonal evolution [
46,
47]. These findings were also corroborated on clinical grounds [
48,
49] and by genetic research [
50,
51,
52,
53,
54]. For example, the KRAS mutation, the most frequently found driver mutation of PDAC, has also been frequently found in intraductal carcinoma [
55,
56].
5. Precursor Lesions
The precursor lesions of pancreatic cancer can be divided into the following groups [
61,
62]:
-
Pancreatic intraepithelial neoplasias (PanIN), which are usually flat, and importantly, they are non-invasive. They are classified into four categories based on the degree of dysplasia [
63]: PanIN-1A, PanIN-1B, PanIN-2, and PanIN-3.
-
Intraductal papillary mucinous neoplasms (IPMN), which are usually large, mucin-producing epithelial lesions originating from the main pancreatic duct or major branch ducts. Their size, which is bigger than PanIN precursor lesions, makes them easy to detect by conventional imaging techniques.
PanIN and IPMN are considered neoplasias. However, both can be subdivided into low-grade and high-grade dysplasia. While this last subgroup is usually surgically resected, the first group is amenable to follow-up observation [
64].
6. Early Diagnosis
The classic description sets two conditions:
- (1)
-
a tumor with a diameter of less than three centimeters that
- (2)
-
has not metastasized.
To these two conditions, we have added a third: lack of involvement of critical vessels.
The metastasis criteria are based on clinical grounds, and it would not be impossible for sub-clinical microscopic metastasis to be present.
The redefinition of early diagnosis consists of achieving a diagnosis before the neoplasia becomes invasive. Following the criteria of the International Cancer of the Pancreas Consortium, early diagnosis “should detect and treat T1N0M0 margin-negative PC and high-grade dysplastic precursor lesions” [
67].
7. Diagnosis and Surgical Treatment
The clinical diagnosis of pancreatic cancer at an early stage is usually difficult because:
- (a)
-
the disease is asymptomatic in the early stage;
- (b)
-
the organ is hidden in the retroperitoneum;
- (c)
-
there are no reliable early tumor markers;
- (d)
-
the existing markers are not sufficiently specific to differentiate benign from malignant disease;
- (e)
-
imaging techniques do not always allow the diagnosis of small surgically resectable cancers, and they are expensive;
- (f)
-
pre-invasive neoplasias are frequently beyond the abilities of imaging techniques, and usual biomarkers are not increased.
Classic and new targeted treatments have almost no impact on pancreatic cancer outcomes, while early diagnosis, when surgery is possible, shows a significantly improved overall survival. Approximately 20% of patients in which surgery is possible will be alive after 5 years [
11]. The percentage is somewhat higher in patients with a tumor of less than 3 cm in diameter. These findings fully justify the search for early diagnostic methods. If the diagnosis can be achieved before the invasive stage, the results should improve substantially.
Mortality and morbidity of classical duodenopancreatectomy (Whipple’s procedure) have decreased in the last 15 years, and overall survival has also improved in surgical cases. Neoadjuvant treatments have increased the rate of operable PDACs [
68,
69].
Due to the need for imaging studies in PC it is almost inevitable to include them in any screening method. Their cost is high, and therefore the population to be screened should be limited to high-risk individuals.
8. Pancreatic Cancer Risk
Age: The first risk factor to analyze should be age. It is highly infrequent to find pancreatic cancer in people under 50 [
70]. The median age at diagnosis in the white population is 75 years [
71]. The median age in the USA is 71 years [
72]. However, native African populations showed a median age of 55.7 years, while African Americans showed a median age of 66.7 [
73,
74].
Race: African Americans have a 20% higher risk than the white population [
76].
Chronic pancreatitis: Chronic pancreatitis represents an important increase in the risk for pancreatic cancer.
Hereditary factors: There is a small group of patients, approximately 10%, in which many cases can be identified in the same family: family clustering. Individuals that belong to families in which two or more cases were identified have a higher risk for PC [
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89].
Cigarette smoking: Most publications agree that cigarette smoking represents an important risk factor for PDAC [
92,
93,
94,
95,
96,
97].
Diabetes: PDAC is more frequent in diabetic individuals. Type 2 diabetes mellitus has been increasing in recent years, and some authors believe that this is one of the causes of the increase in pancreatic cancer [
107]. The risk is increased by 1.5 to 2 fold [
108].
Families with hereditary cancer predisposition syndrome: in addition to familial cancer, there is a group of hereditary mutations that predispose patients to cancer in general and for certain types of cancer in particular. Some of these syndromes also predispose patients to pancreatic cancer.
Unexpected weight loss (UWL) (decrease in body weight of 5% or more). In a retrospective cohort study, 63,973 patients with UWL 1375 (2.2%) were found to have cancer within 2 years. On average, the patients were diagnosed with cancer after 180 days. PDAC was found in 5.3% of male cancer patients and 5.8% in females [
121].
9. High-Risk Population Screening
9.1. Biochemical Screening
CA19-9 (CARBOHYDRATE ANTIGEN 19-9): this is the only serum biomarker approved by the FDA (United States Food and Drug Administration) for diagnostic purposes [162]. CA19-9 is an antigenic protein that can be defined by monoclonal antibody binding to CA19-9, the tumor surface marker Sialyl-Lewis A. (CA19-9 is a sialylated Lewis blood group antigen). It was discovered in 1979 in colorectal carcinoma and later in pancreatic cancer [163,164].
Osteopontin (OPN) is a secreted glycophosphoprotein which is the product of tumor infiltrating macrophages but not of pancreatic tumor cells. Koopman et al. [172] found that OPN was increased in the serum of all 50 patients with early diagnosis treated surgically and in none of the normal controls. They used a cut-off level of 330 ng/mL and ELISA measurement. They also established that “elevated OPN had a sensitivity of 80% and specificity of 97% for pancreatic cancer. In contrast, only 62% of these patients with resectable pancreatic cancer had elevated CA19-9”.
TIMP-1 (tissue inhibitor of metalloproteases-1): Tissue inhibitors of metalloproteinases (TIMPs) are endogenous protein regulators of the matrix metalloproteinase (MMPs) and ADAMs families [176]. One of these inhibitors is overexpressed in pancreatic cancer, and its serum level is also increased. Its value as a diagnostic tumor marker is lower than that of CA19-9 regarding sensitivity and specificity [177]. It did not improve diagnostic accuracy when added to CA19-9. However, increased TIMP-1 urinary levels in patients with PC allowed the discrimination of healthy controls from patients with PC [178].
MMP2 (MATRIX METALLOPROTEASE 2): is an enzyme that degrades collagen type IV. MMP2 is highly expressed in PC cells, including its stroma [
179].
MUC4: MUC4 is a high molecular weight glycoprotein that is over-expressed in pancreatic cancer tissues but not in pancreatic inflammatory diseases [
180,
181]. Importantly, it can be detected in plasma as well. MUC4 expression increases progressively in advancing states of PC [
182].
MUC5AC: is a glycosylated, high-molecular-weight glycosylated protein expressed quite early in precancerous pancreatic cells [
183]. It may be useful for the pathologist to determine borderline cells, but it is not a serum protein with diagnostic potential.
TPS (tissue polypeptide antigen specific): TPS is a specific epitope of the c-terminal part of human cytokeratin 18. Nine patients with symptomatic PC showed a TPS level above 100 U/L, while the non-oncologic controls had a level below 80 U/L [
184]. Unfortunately, TPS increase is not an early phenomenon in PC because 267 pre-diagnostic PDAC plasma samples obtained years before clinical PDAC diagnosis did not show any rise in TPS [
185].
S600A6: this is a protein of the S600 family that binds Zn[2]
+, Ca[2]
+, and Cu[2]
+ and participates in the regulation of diverse cell functions, many of which are involved in tumor progression. Importantly, S600A6 was found to be over-expressed in pancreatic cancer [
186]. Ohuchida et al. [
187] measured mRNA S600A6 levels in the pancreatic juice of normal and pancreatic cancer individuals. All the cases with cancer were found to have an increased level compared with non-neoplastic patients, including those with chronic pancreatitis. Furthermore, S600A6 was increased in the very early phases of pancreatic carcinogenesis [
188].
PC-594: is the result of a metabolomic search for a biomarker [
189]. PC-594 is a circulating 36-carbon polyunsaturated fatty acid that can be identified in normal serum through mass spectrometry. This fatty acid is significantly decreased in pancreatic cancer (0.76 ± 0.07 µmol/L versus 2.79 ± 0.15 µmol/L in control subjects) [
190]. Sensitivity was 90%, and specificity was 87%. These findings were corroborated by further research [
191].
MIC-1 (macrophage inhibitory cytokine-1): Koopman et al. [
192] found that this cytokine was an early marker that allowed differentiating resectable from non-resectable tumors. Furthermore, it was an independent biomarker not related to CA19-9. They also found that: “
MIC-1 was significantly better than CA19-9 in differentiating patients with pancreatic cancer from healthy controls, but not in distinguishing pancreatic cancer from chronic pancreatitis”.
Hepatocyte growth factor (HGF): HGF is a protein related to the main hallmarks of cancer, participating in proliferation, migration, angiogenesis and drug resistance. It is frequently over-expressed in PDAC tissue samples. Importantly it is increate 10-fold in the serum of patients with PDAC compared with normal controls [
193]. However, there is insufficient evidence that increased serum HGF increase is an early marker. Its receptor, c-Met, is also over-expressed in pancreatic cancer cells [
194].
Inflammatory markers are frequently increased in PDAC and are related to pro-tumoral promotion. They are absolutely unspecific, and their plasma increase only reflects the presence of an inflammatory process, whether of tumoral or non-tumoral origin.
Fibrinogen: While albumin synthesis was not found to be decreased, fibrinogen synthesis was increased many fold in PDAC patients with cachexia [
196]. Inflammatory markers, such as C reactive protein and fibrinogen were increased in almost all the patients with advanced PDAC [
197]. The fibrinogen-to-albumin ratio correlates with the progression of the tumor [
198], but this is a prognostic rather than a diagnostic biomarker [
199]. Serum fibrinogen degradation products are also increased in pancreatic cancer, but they are not more sensitive than CA19-9 [
200].
C-reactive protein (CRP): CRP, an important inflammatory marker, has a predictive value in PDAC progression, but it is not a diagnostic marker [
201,
202] or an early increased marker.
Interleukin-6 (IL6): This inflammatory marker is increased in chronic pancreatitis and PDAC, but importantly this increase is significantly higher in PDAC [
204].
9.2. Image Screening
Although imaging may seem an infallible and perfect diagnostic method for the detection of early tumors, this is not so. In the first place, it is fallible because very small tumors are difficult to find by methods such as computed tomography, magnetic resonance cholangiopancreatography and ultrasound studies. By the time the tumors are visible, they are not so small.
Imaging and, eventually, endoscopy are very useful for determining unresectability. However, they are not very effective in diagnosing a small tumor of a few millimeters or an intraductal neoplasia that can be treated surgically.
The other problem is that it is difficult to distinguish small benign lesions from early malignant developments. Although somewhat more accurate, endoscopic ultrasound is a more complex and costly procedure. Endoscopic ultrasound-guided fine-needle aspiration biopsy can be considered the standard procedure for diagnosis in case of doubts. The problem is that this intervention is only feasible after a suspicious image shows up in some other image-based diagnostic procedure.
10. Screening Populations with a Very-High-Risk Level (Score above 75)
Pancreatic cancer has a low incidence in the general population; therefore, indiscriminate general screening is not an option. On the other hand, effective screening methods should be used in very high-risk populations.
This group of patients, mainly individuals from families with frequent pancreatic cancer, or with known cystic lesions, or hereditary cancer predisposition syndromes, are screened/followed up in first-world countries with:
- (a)
-
MRI or MRI cholangiopancreatography and
- (b)
-
Endoscopic ultrasound (EUS) with or without fine needle aspiration/biopsy
Diverse schemes can be followed. A frequently used screening program can consist of magnetic resonance imaging once a year, followed by additional investigations if there are abnormal findings [
208]. With this approach and with a follow up of 262 patients for a little over four years, three pancreatic cancers were detected. In one case, the tumor recurred after surgery and the other two developed metastasis. At first impression, this scheme did not detect malignancies early enough.
Another scheme used CT scan and EUS. If EUS showed alterations, fine needle aspiration or biopsy and endoscopic retrograde cholangiopancreatography were performed [
209]. This method allowed the identification of eight patients with pancreatic cancer in a population of 78 high-risk patients. This means that the follow up identified 10% of an asymptomatic high-risk population as harboring a malignant tumor.
11. Liquid Biopsy (LB) for Pancreatic Cancer Screening
Malignant tumors and also their metastases can release cells and parts of the cells that can be found in blood and other biological fluids, which can be useful for diagnostic purposes. Among these released materials, it is possible to find circulating tumor cells (CTCs), circulating cell-free nucleic acids (cfDNA and cfRNAs), and circulating extracellular vesicles: exosomes.
cfDNA is increased in cancer patients in general [
229,
230] and in PDAC in particular [
231,
232].
cfDNAs reach the blood stream through cell turnover or cell death [
233].
Methodology and results were recently reviewed by Heredia-Soto et al. [
234] and Kumar Yadav et al. [
235]. Although interest in the subject seems to be a new development, Mandel and Metais identified circulating DNA in 1948 [
236,
237]. But the actual initiators of its research and development for oncological purposes were Sorensen et al. [
238].
They have not been systematically introduced in clinical practice regarding pancreatic cancer as yet. The analysis of these materials requires sequencing or other molecular methods to identify specific mutations that can be found in pancreatic cancer. These mutations usually are in the
KRAS,
CDKN2A,
TP53 and
SMAD4 genes. However, these mutations can also be found in non-malignant diseases or low-grade intraductal neoplasias [
239].
12. Promoter Methylation Status of Genes in cfDNA
ADAMTS1 and BNC1. ADAMTS1 is a disintegrin and metalloproteinase with thrombospondin motifs 1 protein; BNC1 is the gene that codes for the zinc finger protein basonuclin-1. In 2019, Eissa et al. [
242] showed that the methylation status of two genes in cell-free DNA, ADAMTS1 and BNC1, was highly sensitive and specific for early PC diagnosis.
SPARC (Secreted Protein Acidic and Rich in Cysteine), UCHL1 (ubiquitin carboxy-terminal hydrolase L1), PENK (proenkephalin), and NPTX2 (neuronal pentraxin 2) are four genes that Sing et al. [
243] have found with methylated promoter regions in cfDNA in patients with pancreatic cancer. SPARC has been shown to be an early marker that permits the differential diagnosis between PC and chronic pancreatitis. Simultaneous high mutilation of SPARC and NPTX2 was found in metastasized PC. UCHL1 methylation correlated with advanced disease. In conclusion, SPARC is an early marker as long as the other genes are not promoter methylated.
Those that may be useful for early diagnosis are:
- ♦
-
Promoter methylation of BNC1 and ADAMTS1.
- ♦
-
Small mutant fragments of cfDNA [
246].
- ♦
-
According to Berger et al. [
247], the total amount of cfDNA may discriminate between patients having early pancreatic cancer or a preinvasive lesion and normal subjects.
- ♦
-
CancerSEEK is a screening multi-test that studies ctDNA (16 genes including
KRAS) and eight cancer-associated proteins in blood: (carbohydrate antigen 125 (CA-125), CA19-9, CEA, HGF (hepatic growth factor), myeloperoxidase, prolactin, OPN, tissue inhibitor of metalloproteinases 1 (TIMP-1)). CancerSEEK was developed by Cohen et al. [
245] as a screening system for different tumors (ovary, liver, esophagus, pancreas, gastric, colorectal, lung, and breast cancer) at relatively early stages. The sensitivity was 70% for PC, and the specificity was high [
248].
13. Circulating Exosomes
The circulating exosomes have the highest potential for PC screening because [
249]:
- (1)
-
They are permanently shed from normal and tumor cells.
- (2)
-
Shedding from tumor cells is more copious.
- (3)
-
Identifying the message they are carrying can be diagnostic for PC.
- (4)
-
No interventional procedure beyond blood sampling is involved.
- (5)
-
They can rule out inflammatory diseases of the pancreas.
- (6)
-
The genetic signature of the cancer can be obtained in many cases.
- (7)
-
Experimental evidence had shown a very high sensitivity, close to 100%.
- (8)
-
Exosome release is increased in malignant cells [
250,
251].
The main drawback is that a somewhat sophisticated laboratory is needed.
Exosomes are simple membrane particles generated through the endolysosomal pathway. They are regularly produced by normal and cancer cells and released into the extracellular matrix from where they enter the circulatory system. They represent a complex mechanism of intercellular communication. Exosomes carry instructions in the form of nucleic acids, proteins (including immunoinhibitory proteins [
252]), and metabolites.
14. Conclusions
Regarding screening methods, the following conclusions were reached:
- (1)
-
Serum biomarkers appear too late in pancreatic ductal adenocarcinoma and are therefore ineffective for timely diagnosis. Despite this limitation, using a battery of markers can probably identify tumors in a stage when they are still candidates for surgery.
- (2)
-
Imaging alone with MRI does not allow early diagnosis, and it is not very effective for screening purposes.
- (3)
-
The association of endoscopic ultrasound and CT scan or MRI imaging (and eventual fine needle aspiration when there are lesions) seems to yield the most reliable results and can contribute to early diagnosis of pancreatic malignancies.
- (4)
-
Pancreatic juice study alone is insufficient for diagnosis, but it may represent an important complement to other methods.
- (5)
-
Circulating cancer cells, free DNA and exosomes have not achieved clinical status yet, and more information is required in this regard. However, miRNAs in circulating exosomes can be useful for early diagnosis. Glypican-1 in exosomes require further testing.
- (6)
-
For the time being, EUS associated with imaging studies is the most reliable screening method.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15184430