Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Plukenetia volubilis Linneo or Sacha Inchi (SI), a traditional natural remedy indigenous to Peru and Brazil, has garnered global attention due to its exceptional nutritional composition. Its protective effects against various non-communicable diseases, notably cardiovascular disease (CVD), have become a subject of interest in recent research.
- inflammation
- obesity
- Plukenetia volubilis Linneo
- sacha inchi
1. Effects of SI on Dyslipidemia
Within the spectrum of CVD risk factors, dyslipidemia has been identified as the most potent contributor [1]. Elevated serum levels of total cholesterol (TC), LDLc, and triglycerides (TG), or a decrease in serum high-density lipoprotein cholesterol (HDLc) levels are well-established risk factors for CVD [2]. The beneficial effects of SI on cholesterol levels have been confirmed in animal and clinical studies. Obese rats fed 2.5 mL of SI emulsion oil with different ω-3 content (0.2 g and 0.5 g ω-3/day) for eight weeks showed decreases in TC, TG, and LDLc levels, and increases in HDLc levels [3]. The treatment of patients with hypercholesterolemia with 5 or 10 mL of SI oil (contains 2 g and 4 g ω-3/day, respectively) for 16 weeks also caused a significant reduction in their total cholesterol and LDLc and increment in their HDLc levels [4].
SI oil is also helpful in preventing dyslipidemia in healthy individuals. Consumption of 10 or 15 mL of SI oil by healthy individuals for 16 weeks was found to significantly reduce their serum TC and LDLc levels and increase their HDLc levels [5]. In a randomized crossover clinical trial, the consumption of 15 mL SI oil alongside a high-fat meal reduced the postprandial increase in TC levels and the inflammatory marker interleukin- 6 (IL-6) in metabolically healthy men. However, no significant differences were observed in their HDLc and TG levels, which is most likely due to the short duration of the intervention [6]. In contrast, SI oil intake with a high-fat meal reduced the postprandial increase in IL-6 but failed to reverse the postprandial cholesterol increase in metabolically unhealthy men. This shows that the effect of SI on postprandial lipid levels following a high-fat meal depends on the individual’s metabolic status [6].
SI exerts antihyperlipidemic activity in vitro, mainly via enzymatic inhibitory reaction [7]. The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is a rate-controlling enzyme in the mevalonate pathway of cholesterol biosynthesis. HMG-CoA reductase inhibitors, such as statins, have long been used to efficiently treat hypercholesterolemia. However, its multisystemic adverse effects necessitate the discovery of plant-based HMG-CoA reductase inhibitors with minimal side effects [8][9]. An in vitro study showed that 125 µg/mL of SI nutshell hot water extract inhibited HMG-CoA reductase activity by 65% [10]. Contrary to the mechanism of action of statins, SI nutshell extract exhibits a precise and effective non-competitive inhibition pattern [10][11]. In addition, there was a 38.1% reduction in cholesterol esterase activity using a similar SI extract concentration [10]. The inhibition of cholesterol esterase activity interferes with cholesterol absorption and transport into enterocytes, which contributes to the lipid-lowering effect of SI [12]. However, SI baby nut and leaf hot water extracts and SI nut oil did not show any HMG-CoA reductase or cholesterol esterase inhibitory effects [10].
Recent studies have identified a close relationship between the gut microbiome and dyslipidemia [13][14]. High dietary fat induces dysbiosis of the gut microbiota, resulting in lipid dysmetabolism [15]. SI oil consumption reversed gut microbiota dysbiosis in high-fat diet (HFD)-fed rats, thus improving their TC, TG, and LDLc levels [16]. One of the mechanisms is through the influence of the gut microbiota metabolome on bile acid composition. Bile acids regulate hepatic lipid metabolism by facilitating lipid absorption [17][18]. HFD altered gut microbiota metabolome and bile acid composition in the small intestine, leading to elevated levels of taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), cholic acid, and glycocholic acid, which contribute to lipid dysmetabolism and hyperlipidemia. Supplementation with 0.5–1.5 mL/kg SI oil for eight weeks reversed the changes in gut microbiota metabolome and bile acid composition, and improved lipid dysmetabolism in HFD-fed rats [16]. Table 1 and Figure 1 summarize the current knowledge on the protective effects of SI on lipid metabolism and dyslipidemia.
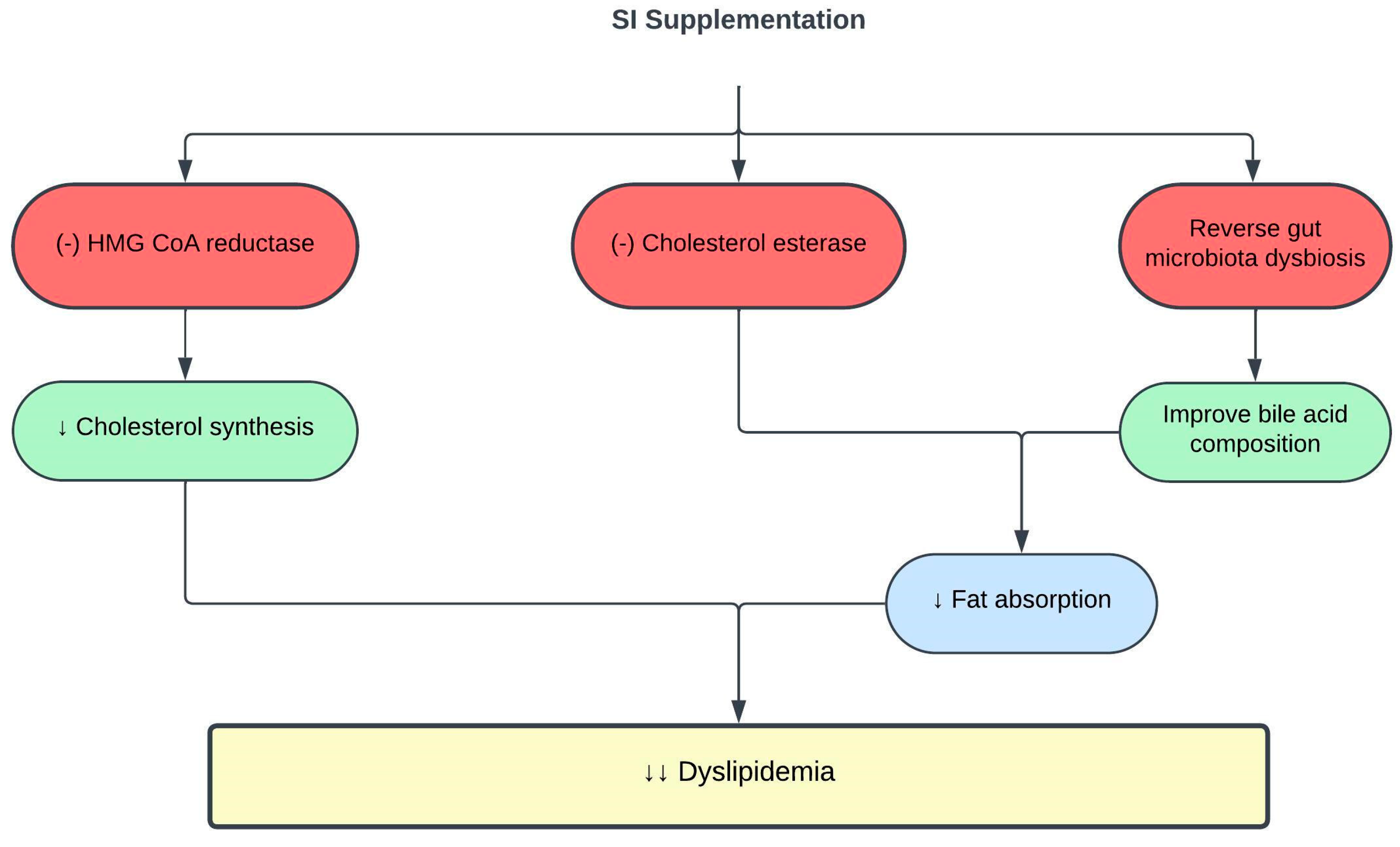
Figure 1. Protective effects of SI on dyslipidemia. (-), inhibit; ↓, decrease; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A.
Table 1. Effects of SI on lipid metabolism and dyslipidemia.
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 5 or 10 mL for 16 weeks | Healthy adults | ↓ TC and LDLc ↑ HDLc |
[5] |
| SI oil | 15 mL | Metabolically healthy and unhealthy men given high-fat meal | ↓ postprandial TC and IL-6 in metabolically healthy men ↓ postprandial IL-6 in metabolically unhealthy men |
[6] |
| SI oil | 5 or 10 mL (contains 2 g or 4 g ω-3/day) for 16 weeks | Hypercholesterolemic patients | ↓ TC and LDLc ↑ HDLc |
[4] |
| SI emulsion oil | 2.5 mL (contains 0.2 g or 0.5 g ω-3/day) for 8 weeks | Obese rats | ↓ TC, TG, and LDLc ↑ HDLc |
[3] |
| SI oil | 0.5–1.5 mL/kg for 8 weeks | Obese rats | ↓ TC, TG, and LDLc Reverse gut microbiota dysbiosis and metabolome Improve bile acid compositions |
[16] |
| SI nutshell, baby nut and leaf hot water extracts SI nut oil |
125 µg/mL | In vitro enzyme inhibitory assays | Only SI nutshell hot water extract ↓ HMG-CoA reductase and cholesterol esterase activities | [10] |
Abbreviations: ↑, increase; ↓, decrease; HDLc, high-density lipoprotein cholesterol; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme; IL-6, interleukin-6; LDLc, low-density lipoprotein cholesterol; TC, total cholesterol.
2. Effects of SI on Obesity
CVD is associated with obesity and high visceral fat deposition [19]. SI exerts anti-obesity effects in vivo. High-fat diet (HFD)-fed Sprague Dawley rats supplemented with 0.5–1.5 mL/kg SI oil for eight weeks showed significant reduction in their adipocyte size, hepatic steatosis, and inflammation. This is associated with increased hepatic lipase expression following SI oil supplementation [16]. Hepatic lipase is an essential lipolytic enzyme that facilitates lipoprotein uptake in the de novo lipid synthesis pathway [20]. Furthermore, SI oil suppressed the production of lysophosphatidylcholine (LysoPC) and lysophosphatidylethanolamine (LysoPE) [16], which have been identified as pro-inflammatory phospholipids found in patients with hyperlipidemia and HFD-induced obesity [21]. In addition, SI oil decreased the expression of hepatic phosphatidylglycerol phosphate synthase 1 (PGS1) in HFD-fed rats [16]. PGS1 catalyzes the production of phosphatidylglycerol, which is a potent inhibitor of lipolysis [22]. Therefore, the inhibition of PGS1 by SI oil reduced phosphatidylglycerol production and improved lipolysis [16].
Pancreatic lipase has been identified as a key enzyme in systemic lipid digestion and absorption [23]. Its inhibition has gained significant interest as an efficient method to reduce obesity [24]. Interestingly, SI meal-derived peptides, a by-product generated from SI oil, exhibited strong pancreatic lipase inhibitory activity, hypothetically by competitive binding to the pancreatic lipase catalytic sites [25]. Pancreatic lipase inhibitory peptides were previously demonstrated to reduce the amount of intracellular fat accumulations by neutralizing ROS production in oleic acid-induced HepG2 cells [25]. Meanwhile, the SI protein isolate showed high antioxidative activity, which might contribute to its pancreatic lipase inhibitory effect [26]. A similar lipase inhibition pattern was also observed with SI husk aqueous ethanol extract [27]. The lipase inhibitory effect of SI may be attributed to the synergistic actions of its various phenolic and active peptide compounds [27].
Obesity leads to complications such as dyslipidemia and CVD through mechanisms involving oxidative stress, low-grade inflammation, and cellular hypoxia [28]. Oxidative stress promotes lipid and protein oxidation [29], as evidenced by the production of the end-products; malondialdehyde (MDA) and advanced protein oxidation products (AOPP) [30][31][32]. Treatment of obese rats with the emulsion of SI oil (2.5 mL, contains 0.25 g and 0.5 g ω-3/day) reduced MDA and AOPP levels in the rat serum [3]. SI oil emulsion also enhanced antioxidant capacity by stimulating the activity of the antioxidant enzyme catalase [3][33]. Furthermore, SI oil emulsion attenuated inflammation in obese rats, as evidenced by reduced levels of the proinflammatory cytokines IL-6 and tumor necrosis factor-α (TNF-α) [3]. Collectively, the findings showed that the antioxidative and anti-inflammatory activities of SI contribute to its anti-obesity effect.
The pro-inflammatory state in obesity is associated with changes in adipokine release by adipose tissues, such as leptin and adiponectin [34][35]. Leptin regulates body fat composition by augmenting energy expenditure and inhibiting appetite [36]. However, leptin resistance and hyperleptinemia in obesity promote hunger, increase food consumption, and induce inflammation [37]. Meanwhile, adiponectin is an anti-inflammatory adipokine, and its level is reduced in obesity [38]. Interestingly, the emulsion of SI oil reduced leptin levels and increased adiponectin levels in obese rats [3]. Increased adiponectin levels are also responsible for the hypolipidemic effect of SI, as adiponectin activates lipoprotein lipase to degrade circulating TG [39]. The positive effects of SI oil on leptin and adiponectin levels were mediated via increased expression of peroxisome proliferator-activated receptor alpha (PPAR-α), which is a crucial transcription factor that regulates fatty acid metabolism and oxidative stress [33]. Table 2 and Figure 2 summarize the current knowledge on the protective effects of SI on obesity. Exploring the impact of SI supplementation on body mass index, body fat composition, and basal metabolic rate in individuals with obesity presents an intriguing opportunity since there has been no prior research delving into this subject.
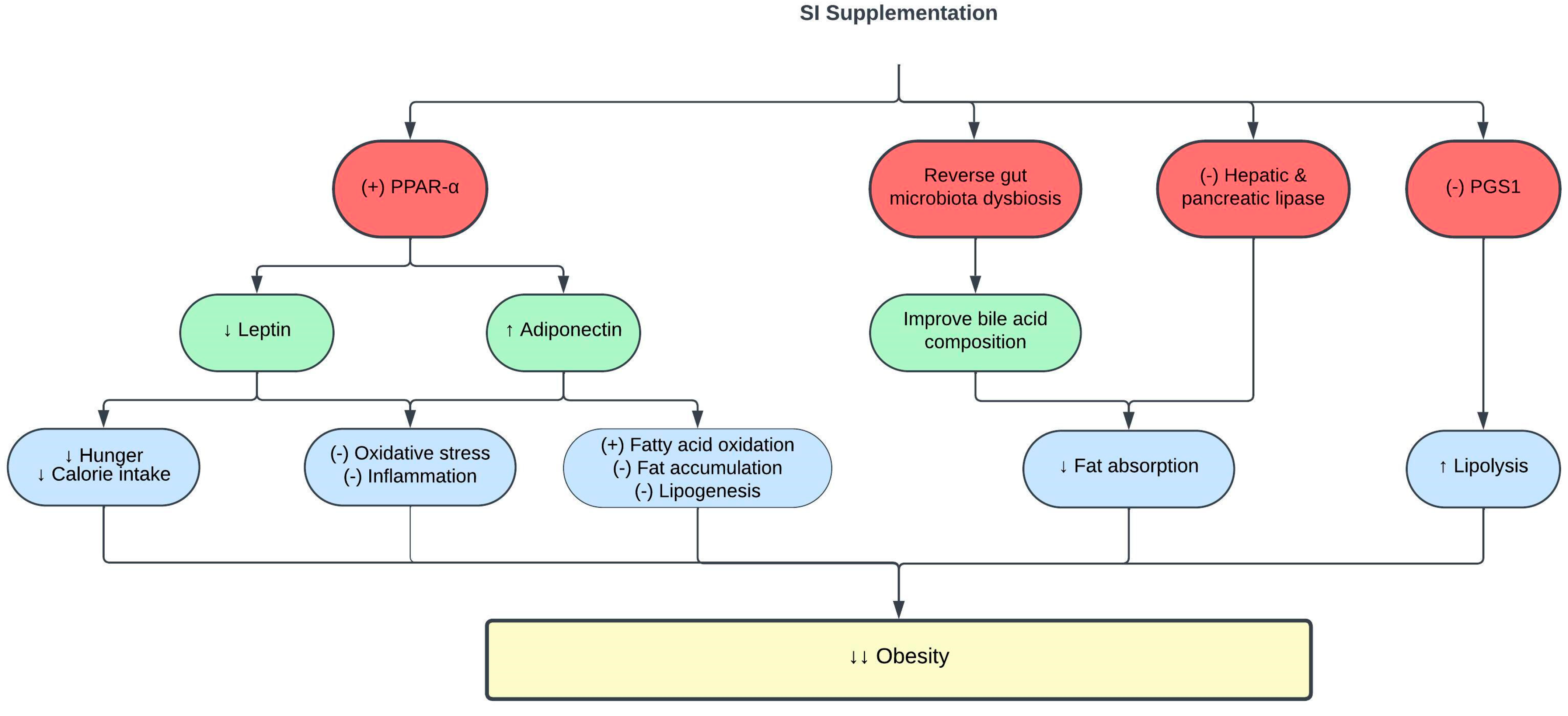
Figure 2. Protective effects of SI on obesity. (-), inhibit; (+), stimulate; ↑, increase; ↓, decrease; PGS1, phosphatidylglycerol phosphate synthase 1; PPAR-α, peroxisome proliferator-activated receptor alpha.
Table 2. Effects of SI on obesity.
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil emulsion | 2.5 mL (contains 0.25 g and 0.5 g ω-3/day) for 8 weeks | Obese rats | ↓ MDA and AOPP ↑ catalase activity ↓ IL-6 and TNF-α ↓ leptin ↑ adiponectin ↑ PPAR-α |
[3] |
| SI oil | 0.5–1.5 mL/kg for 8 weeks | Obese rats | ↓ mean adipocyte size ↓ hepatic steatosis, hepatic lipase activity and inflammation ↓ PGS1 expression ↑ lipolysis |
[16] |
| SI meal-derived peptides | 0.1–0.5 mM | In vitro enzyme inhibitory assay Oleic acid-induced HepG2 cells |
↓ pancreatic lipase activity ↓ intracellular fat accumulation and ROS levels in HepG2 cells |
[25] |
| SI husk aqueous ethanol extract | 0.4 mg/mL | In vitro enzyme inhibitory assay | ↓ lipase activity | [27] |
Abbreviations: ↑, increase; ↓, decrease; AOPP, advanced protein oxidation products; IL-6, interleukin-6; MDA, malondialdehyde; PPAR-α, peroxisome proliferator-activated receptor alpha; PGS1, phosphatidylglycerol phosphate synthase 1; TNF-α, tumor necrosis factor alpha.
3. Effects of SI on Glucose Metabolism and Diabetes
Hyperglycemia is the hallmark of diabetes mellitus, which is also a risk factor for CVD [40]. The inhibition of α-glucosidase, α-amylase, and dipeptidyl peptidase (DPP-IV) is a common method used to assess the potential antidiabetic properties of natural products. α-glucosidase inhibitors block enzymes such as glucoamylase, sucrase, maltase, and isomaltase found at the brush border of the intestinal epithelium. This inhibition prevents the absorption of carbohydrates in the small intestine, reducing postprandial hyperglycemia [41]. On the other hand, α-amylase inhibitors hinder the breakdown of α-(1–4)-d-glucosidic linkages in starch, thereby decreasing carbohydrate digestion and absorption in the gastrointestinal tract and consequently lowering blood glucose levels [42]. DPP-IV inhibitors block the degradation of incretin hormones, specifically glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Increased GLP-1 and GIP levels stimulate insulin release, inhibit glucagon secretion, and delay gastric emptying, thus improving postprandial glucose levels.
It has been reported that 0.035 mg/mL of SI husk and shell extracts exhibited strong α-glucosidase and α-amylase inhibitory activities [27]. Correspondingly, 25 µg/mL of SI essential oil exhibited robust α-amylase inhibition [43]. The potent α-glucosidase and α-amylase inhibitory effects are linked to the phenolic content in SI [44][45][46][47]. Furthermore, SI meal-derived peptides demonstrated potent DPP-IV inhibitory activity in vitro, which was further validated with increased glucose consumption by palmitic acid-induced insulin resistant HepG2 cells [48]. It is important to emphasize that α-amylase, α-glucosidase, and DPP-IV inhibitory assays serve as valuable screening tools to evaluate the antidiabetic potential of natural products. However, it is crucial to complement these results with further in vivo studies to validate their antidiabetic activity.
The most employed in vivo model for inducing diabetes in laboratory animals involves the chemical ablation of pancreatic β-cells using streptozotocin (STZ). STZ functions as a toxic glucose analog that selectively accumulates within pancreatic β-cells via the GLUT2 glucose transporters located on the plasma membrane. Once taken up by β-cells, STZ initiates oxidative stress and DNA alkylation, ultimately leading to pancreatic β-cell necrosis, reduced insulin production, and hyperglycemia [49][50][51]. Furthermore, it is important to note that GLUT2 transporters are not limited to pancreatic β-cells but are also found in the epithelial cells of the kidneys and hepatocytes. Therefore, the administration of STZ may lead to kidney and liver toxicity, in addition to its capacity to harm pancreatic β-cells [52]. A single, high dose of STZ injection induces pancreatic β-cell damage and diabetes in rats, mimicking type 1 diabetes [53]. A low dose of STZ injection combined with HFD to induce hyperglycemia and insulin resistance is a method to mimic type 2 diabetes in rats [54].
The treatment of type 2 diabetic rats with 0.5–2 mL/kg of SI oil for five weeks significantly reduced fasting blood glucose levels and improved insulin sensitivity indices and glucose tolerance in a dose-dependent manner [55]. Insulin plays a pivotal role in regulating glucose metabolism by activating the insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. The dysfunction of insulin receptors (IR) and subsequent impairment of downstream signaling are critical factors in the onset of insulin resistance [56]. This condition, which is characterized by the inability of cells to respond effectively to insulin, contributes to heightened hepatic gluconeogenesis and glycogenolysis. Ultimately, these processes culminate in elevated blood sugar levels, leading to hyperglycemia in individuals with diabetes [57]. SI enhances hepatic insulin sensitivity by downregulating IR-β and stimulating IRS-1 and Akt. Moreover, SI also inhibited the activities of glucose-6-phosphatase (G-6-Pase) and phosphoenolpyruvate carboxykinase-1 (PCK-1) in the liver of diabetic rats [55]. Reduced G-6-Pase and PCK-1 levels suppress hepatic gluconeogenesis and enhance glycogenesis, thereby reducing blood glucose levels [58]. The bioactive compounds present in SI, including ω-3 fatty acids, β-sitosterols, and flavonoids, contribute to its insulin sensitizing effect [55].
Extensive studies have shown the role of intestinal microflora in various stages of diabetes progression [59][60]. An intimate relationship exists between gut microbiota dysbiosis and diabetes [61][62][63]. Pathogenic bacteria in patients with diabetes were elevated in parallel with pro-inflammatory cytokines such as IL-6. These findings denote the involvement of gut microbiota dysbiosis and inflammation in the pathogenesis of diabetes. Interestingly, 400 mg/kg SI leaf water extract treatment for six weeks exerted prebiotic activity in type 1 diabetic rats by diversifying beneficial intestinal bacteria, primarily Akkermansia, Parabacteroides, Bacteroides, and Alloprevotella, while simultaneously suppressing diabetes-related bacteria, including Lactobacillus, Ruminococcaceae, Ruminiclostridium, and Oscillibacter [64]. The amelioration of gut microbiota dysbiosis with SI treatment led to a reduction in blood glucose levels, and improved glucose tolerance and insulin resistance in diabetic rats [64].
In a clinical trial, the addition of 15 mL SI oil to a high-fat breakfast attenuated postprandial hyperglycemia and improved insulin sensitivity in healthy individuals with higher baseline triglycerides and glycemic response. In addition, these individuals also showed increased sirtuin 1 (SIRT-1) gene expression in their peripheral blood mononuclear cells 4 h postprandially [65]. The SIRT-1 gene plays a pivotal role in glucose metabolism, and its expression is downregulated in people with obesity and insulin resistance [66][67]. Hence, SI oil improves glycemic control and insulin sensitivity by enhancing SIRT-1 expression. However, to date, there is no clinical trial involving SI supplementation in patients with diabetes.
Diabetes leads to target organ damage, and the liver is one of the target organs of diabetes complications [68]. SI oil supplementation protected the liver from diabetes-induced liver damage, as evidenced by reduced serum alanine transaminase and aspartate transaminase (AST) levels, and improved hepatic histopathological changes [55]. The underlying mechanisms of such findings were attributed to the antioxidative and anti-inflammatory effects of SI, as treatment with SI oil reduced the oxidative stress marker (MDA), enhanced the antioxidant enzyme activity (superoxide dismutase, catalase and glutathione peroxidase) and reduced the inflammatory markers (TNF-α and IL-6) in the liver of diabetic rats [55]. However, to date, no studies have investigated how SI impacts other diabetes-related target organ damage, such as those affecting the kidneys, heart, and blood vessels. A summary of the protective effects of SI on glucose metabolism and diabetes is shown in Table 3 and Figure 3.
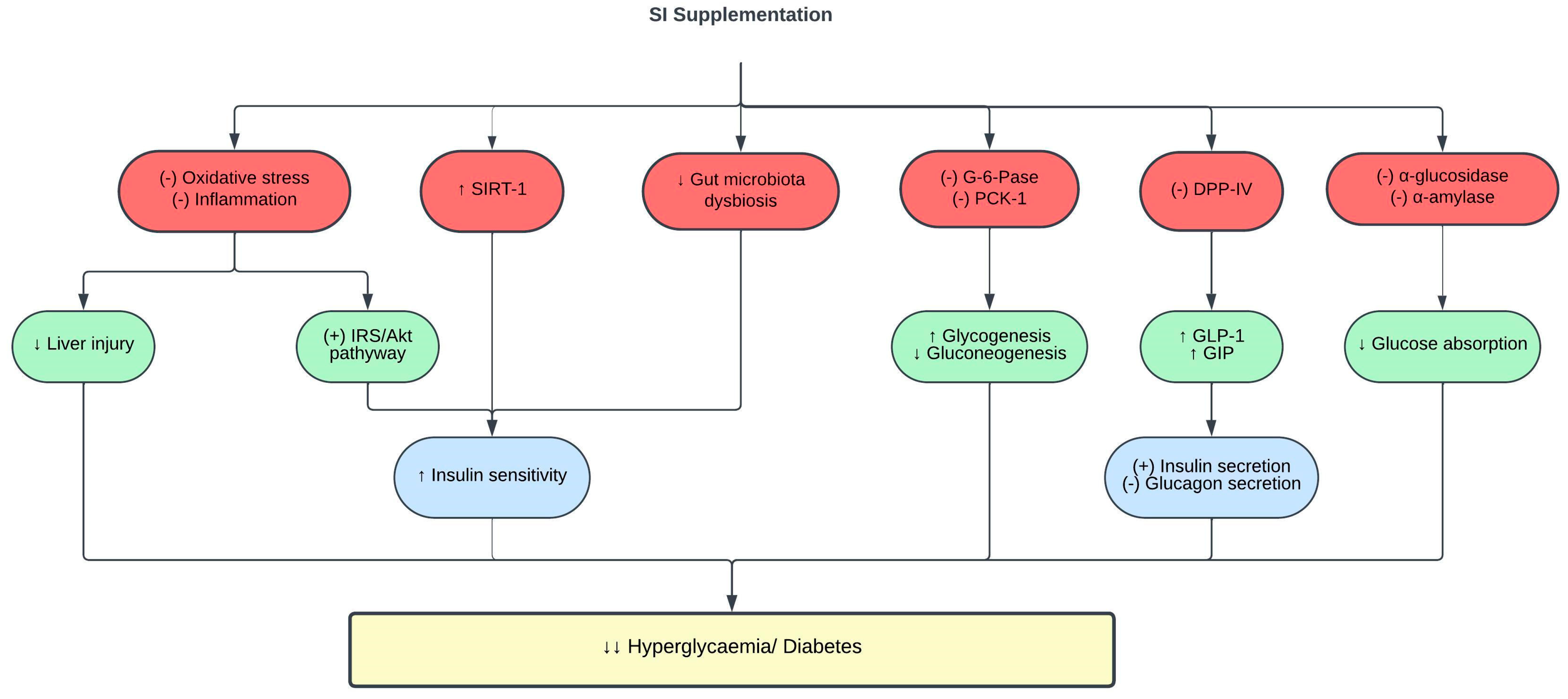
Figure 3. Protective effects of SI on glucose metabolism and diabetes. (-), inhibit; (+), stimulate; ↑, increase; ↓, decrease; Akt, protein kinase B; DPP-IV, dipeptidyl peptidase IV; G-6-Pase, glucose-6-phosphatase; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; IRS, insulin receptor substrate; PCK-1, phosphoenolpyruvate carboxykinase-1; SIRT-1, sirtuin-1.
Table 3. Effects of SI on blood glucose and diabetes.
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 15 mL | Healthy adults given high-fat breakfast | ↓ postprandial hyperglycemia ↑ insulin sensitivity ↑ SIRT-1 expression in healthy adults with higher baseline triglycerides and glycemic response |
[65] |
| SI leaves water extract | 400 mg/kg for 6 weeks | Type 1 diabetic rats | ↓ FBS ↑ insulin sensitivity and glucose tolerance ↓ gut microbiota dysbiosis |
[64] |
| SI oil | 0.5–2 mL/kg for 5 weeks | Type 2 diabetic rats | ↓ FBS ↑ insulin sensitivity indices and glucose tolerance ↑ IRS-1 and Akt ↓ IR-β ↓ G-6-Pase and PCK-1 activities ↑ hepatic glycogen content ↓ AST and ALT ↓ MDA ↑ SOD, CAT and GPX activities ↓TNF-α and IL-6 |
[55] |
| SI husk and shell aqueous ethanol extract | 0.025 mg/mL | In vitro enzyme inhibitory assays | ↓ α-glucosidase and α-amylase activities | [27] |
| SI essential oil | 25 µg/mL | In vitro enzyme inhibitory assays | ↓ α-amylase activity | [43] |
| SI meal-derived peptides | 0.25–0.5 mM | In vitro enzyme inhibitory assay Palmitic acid-induced insulin resistant HepG2 cells |
↓ DPP-IV activity ↑ glucose consumption by HepG2 cells |
[48] |
Abbreviations: ↑, increase; ↓, decrease; Akt, protein kinase B; ALT, alanine transaminase; AST, aspartate transaminase; CAT, catalase; DPP-IV, dipeptidyl peptidase IV; FBS, fasting blood sugar; G-6-Pase, glucose-6-phosphatase; GPX, glutathione peroxidase; IR-β, insulin receptor-β; IRS-1; insulin receptor substrate-1; IL-6, interleukin-6; MDA, malondialdehyde; PCK-1, phosphoenolpyruvate carboxykinase-1; SIRT-1, sirtuin-1; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha.
4. Effects of SI on Blood Pressure
Extensive evidence has consistently established a linear relationship between elevated blood pressure and the risk of CVD [69]. To date, few studies have been conducted to study the blood pressure-lowering effect of SI. Only one clinical trial was conducted to determine the effect of SI on blood pressure in human subjects. Gonzales et al. [5] revealed that clinically healthy adults who consumed 10 and 15 mL of SI oil for four months had reduced systolic and diastolic blood pressure. The reduction in blood pressure was suggested to be due to the LDL-lowering effect of SI oil. Increased LDL levels have been linked to the pathophysiology of hypertension [70][71][72][73]. However, there is no clinical trial involving SI supplementation in patients with hypertension.
The renin angiotensin aldosterone system regulates blood pressure and body fluid homeostasis. Overactivation of this system is implicated in the pathogenesis of hypertension; hence, angiotensin-converting enzyme (ACE) inhibitors are commonly prescribed to treat hypertension [74]. In vitro evaluation using 0.013 mg/mL SI husk and shell extracts demonstrated ACE inhibitory activities [27]. Similarly, SI protein hydrolysates showed strong ACE inhibitory activity at a concentration of 98 µg/mL [75]. This ACE inhibitory effect is correlated with the high phenolic content in SI, especially in the shell [27][76].
Individuals with hypertension may have faulty renal handling of calcium, particularly due to upregulation of the calcium signaling pathway. This leads to increased expression of L-type calcium channels (LTCC) that allow a massive calcium ion influx into the vascular smooth muscle cells, causing vasoconstriction and increasing the blood pressure. In addition, sodium retention and plasma volume expansion have been identified as precursor of hypertension [77]. The Na+/K+-ATPase pump is essential for maintaining the electrochemical gradient of sodium across the cell membrane. In hypertension, the expression of Na+/K+-ATPase is decreased, which contributes to sodium retention and plasma volume expansion [78]. SI shell extract (SISE) exerts its antihypertensive effect in spontaneous hypertensive rats (SHR) and high-salt-diet-fed Wistar-Kyoto (WKY) rats by restoring the expression of LTCC and Na+/K+-ATPase, thus maintaining calcium and sodium homeostasis [79].
Oxidative stress and chronic inflammation are involved in the pathogenesis of hypertension [80]. NO produced by eNOS is an important vasoactive molecule that modulates vascular functions and blood pressure. Continuous release of superoxide exceeding the endogenous antioxidant capacity reduces NO bioavailability [81][82][83]. Reduced NO levels lead to endothelial dysfunction, impaired vasodilation, and elevated blood pressure [84]. SISE decreased the blood pressure of SHR and WKY rats fed a high-salt diet by reducing oxidative stress and inflammation, and increasing the expression of eNOS and NO. Concurrently, the level of 5-methyltetrahydrofolate (5-MTHF), an active form of di- and tetrahydrofolic acid, was found to be increased with SISE treatment [79]. 5-MTHF maintains NO bioavailability by suppressing superoxide production [85].
Multiple studies have linked abnormal gut microbiota to the pathogenesis of hypertension [86][87]. Gut microbiome dysbiosis or imbalance of Firmicutes/Bacteroidetes (F/B) ratio is often correlated with various pathological conditions, including hypertension [88][89]. The gut microbiota of patients and animals with hypertension also showed reduced levels of beneficial bacteria such as Roseburia, and increased levels of harmful bacteria such as Prevotella [90]. One of the mechanisms underlying the antihypertensive effect of SISE is through reshaping of the gut microbiota and metabolome, in which SISE improved the prevalence of Roseburia and dihydrofolic acid levels in the gut and normalized the F/B ratio [79]. A summary of the effects of SI on blood pressure and hypertension is shown in Table 4 and Figure 4.
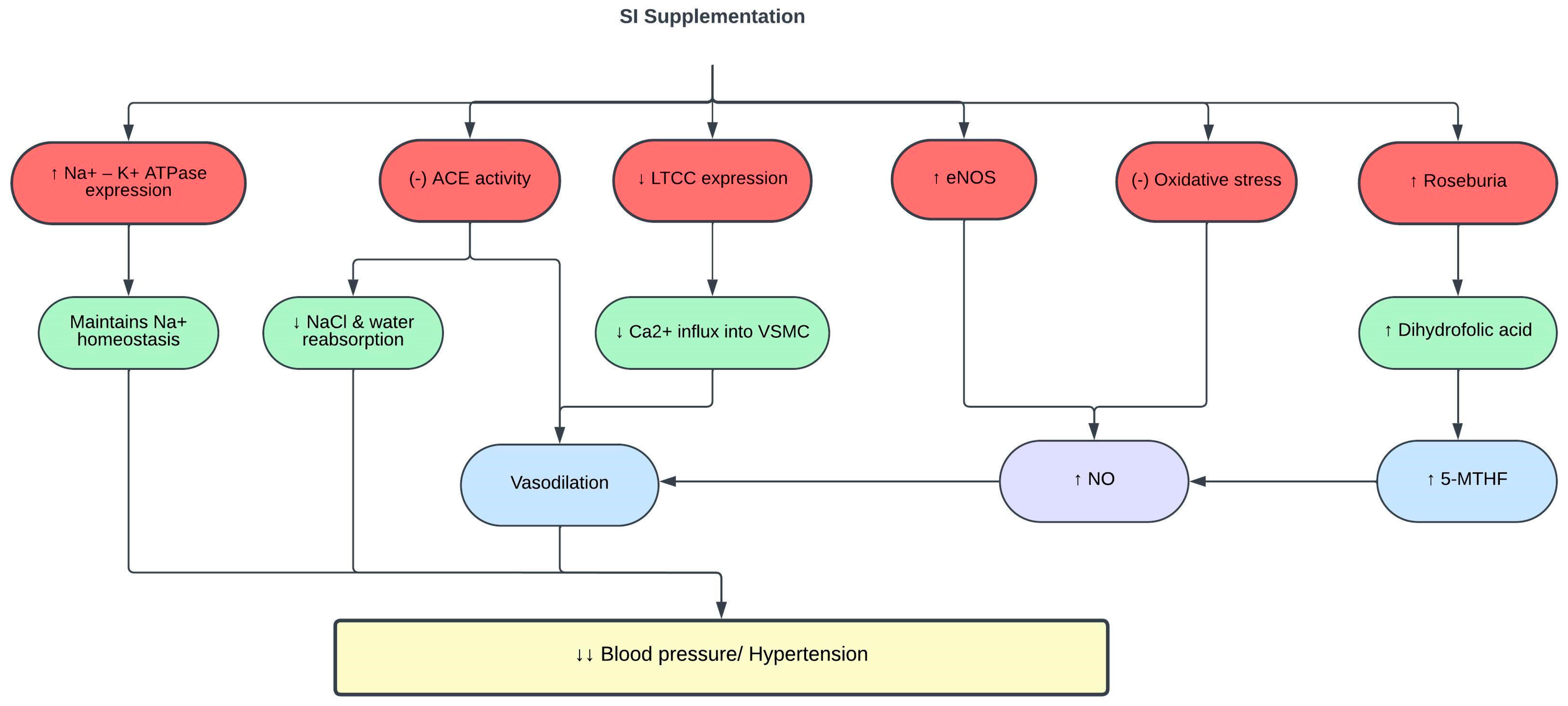
Figure 4. Protective effects of SI on blood pressure. (-), inhibit; ↑, increase; ↓, decrease; 5-MTHF, 5-methyltetrahydrofolate; ACE, angiotensin-converting enzyme; Ca2+, calcium ions; eNOS, endothelial nitric oxide synthase; K+, potassium ions; LTCC, L-type calcium channels; Na+, sodium ions; NaCl, sodium chloride; NO, nitric oxide; VSMC, vascular smooth muscle cells.
Table 4. Effects of SI on blood pressure and hypertension.
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 10 or 15 mL for 4 months | Healthy adults | ↓ SBP and DBP ↓ LDLc |
[5] |
| SI shell extract | 400 mg/kg for 51 days | SHR and WKY rats on high-salt diet | ↓ SBP ↓ LTCC expression ↑ Na+/K+-ATPase expression Restored calcium and sodium homeostasis ↓ MDA ↑ SOD and GSH ↑ eNOS expression ↑ NO ↑ 5-MTHF Reshaped gut microbiota and metabolome, ↑ prevalence of Roseburia and dihydrofolic acid Normalized F/B ratio |
[79] |
| SI protein hydrolysates | 98 µg/mL | In vitro enzyme inhibitory assay | ↓ ACE activity | [75] |
| SI husk and shell aqueous ethanol extract | 0.013 mg/mL | In vitro enzyme inhibitory assay | ↓ ACE activity | [27] |
Abbreviations: ↑, increase; ↓, decrease; 5-MTHF, 5-methyltetrahydrofolate; ACE, angiotensin-converting enzyme; DBP, diastolic blood pressure; eNOS, endothelial nitric oxide synthase; F/B, Firmicutes/Bacteroidetes; GSH, glutathione; LDLc, low-density lipoprotein cholesterol; LTCC, L-type calcium channels; MDA, malondialdehyde; NO, nitric oxide; SBP, systolic blood pressure; SOD, superoxide dismutase.
This entry is adapted from the peer-reviewed paper 10.3390/ph16111588
References
- Ibrahim, N.I.; Naina Mohamed, I. Interdependence of Anti-Inflammatory and Antioxidant Properties of Squalene–Implication for Cardiovascular Health. Life 2021, 11, 103.
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis. 2020, 19, 42.
- Ambulay, J.P.; Rojas, P.A.; Timoteo, O.S.; Barreto, T.V.; Colarossi, A. Effect of the Emulsion of Sacha Inchi (Plukenetia Huayabambana) Oil on Oxidative Stress and Inflammation in Rats Induced to Obesity. J. Funct. Foods 2020, 64, 103631.
- Garmendia, F.; Pando, R.; Ronceros, G. Effect of Sacha Inchi Oil (Plukenetia volúbilis L.) on the Lipid Profile of Patients with Hyperlipoproteinemia. Rev. Peru. Med. Exp. Salud Publica 2011, 28, 628–632.
- Gonzales, G.F.; Gonzales, C. A Randomized, Double-Blind Placebo-Controlled Study on Acceptability, Safety and Efficacy of Oral Administration of Sacha Inchi Oil (Plukenetia volubilis L.) in Adult Human Subjects. Food Chem. Toxicol. 2014, 65, 168–176.
- Alayón, A.N.; Ortega Ávila, J.G.; Echeverri Jiménez, I. Metabolic Status Is Related to the Effects of Adding of Sacha Inchi (Plukenetia volubilis L.) Oil on Postprandial Inflammation and Lipid Profile: Randomized, Crossover Clinical Trial. J Food Biochem 2019, 43, e12703.
- Suwanangul, S.; Aluko, R.E.; Sangsawad, P.; Kreungngernd, D.; Ruttarattanamongkol, K. Antioxidant and Enzyme Inhibitory Properties of Sacha Inchi (Plukenetia volubilis) Protein Hydrolysate and Its Peptide Fractions. J. Food Biochem. 2022, 46, e14464.
- Bellosta, S.; Corsini, A. Statin Drug Interactions and Related Adverse Reactions: An Update. Expert Opin. Drug Saf. 2018, 17, 25–37.
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687.
- Prasongsub, W.; Pimsan, N.; Buranapattarachote, C.; Punturee, K. Anti-HMG-CoA Reductase and Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Nutshell Extract. Thai-J. Cit. Index Cent. (TCI) ASEAN Cit. Index 2021, 54, 18–26.
- da Costa, R.F.; Freire, V.N.; Bezerra, E.M.; Cavada, B.S.; Caetano, E.W.S.; de Lima Filho, J.L.; Albuquerque, E.L. Explaining Statin Inhibition Effectiveness of HMG-CoA Reductase by Quantum Biochemistry Computations. Phys. Chem. Chem. Phys. 2012, 14, 1389–1398.
- Asmaa, B.H.; Ream, N. In Vitro Screening of the Pancreatic Cholesterol Esterase Inhibitory Activity of Some Medicinal Plants Grown in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1432–1436. Available online: www.ijppr.com (accessed on 18 September 2023).
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The Modulatory Effect of Infusions of Green Tea, Oolong Tea, and Black Tea on Gut Microbiota in High-Fat-Induced Obese Mice. Food Funct. 2016, 7, 4869–4879.
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The Gut Microbiota Drives the Impact of Bile Acids and Fat Source in Diet on Mouse Metabolism. Microbiome 2018, 6, 134.
- Yang, Y.; Zhang, Y.; Xu, Y.; Luo, T.; Ge, Y.; Jiang, Y.; Shi, Y.; Sun, J.; Le, G. Dietary Methionine Restriction Improves the Gut Microbiota and Reduces Intestinal Permeability and Inflammation in High-Fat-Fed Mice. Food Funct. 2019, 10, 5952–5968.
- Li, P.; Huang, J.; Xiao, N.; Cai, X.; Yang, Y.; Deng, J.; Zhang, L.H.; Du, B. Sacha Inchi Oil Alleviates Gut Microbiota Dysbiosis and Improves Hepatic Lipid Dysmetabolism in High-Fat Diet-Fed Rats. Food Funct. 2020, 11, 5827–5841.
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and Lipidomics Profiling Reveals Hypocholesterolemic and Hypolipidemic Effects of Arabinoxylan on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 10614–10623.
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50.
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The Clinical Importance of Visceral Adiposity: A Critical Review of Methods for Visceral Adipose Tissue Analysis. Br. J. Radiol. 2012, 85, 1–10.
- Balla, T.; Sengupta, N.; Kim, Y.J. Lipid Synthesis and Transport Are Coupled to Regulate Membrane Lipid Dynamics in the Endoplasmic Reticulum. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158461.
- Fang, L.; Harkewicz, R.; Hartvigsen, K.; Wiesner, P.; Choi, S.-H.; Almazan, F.; Pattison, J.; Deer, E.; Sayaphupha, T.; Dennis, E.A.; et al. Oxidized Cholesteryl Esters and Phospholipids in Zebrafish Larvae Fed a High Cholesterol Diet. J. Biol. Chem. 2010, 285, 32343–32351.
- Kayser, B.D.; Lhomme, M.; Prifti, E.; Da Cunha, C.; Marquet, F.; Chain, F.; Naas, I.; Pelloux, V.; Dao, M.; Kontush, A.; et al. Phosphatidylglycerols Are Induced by Gut Dysbiosis and Inflammation, and Favorably Modulate Adipose Tissue Remodeling in Obesity. FASEB J. 2019, 33, 4741–4754.
- Mukherjee, M. Human Digestive and Metabolic Lipases—A Brief Review. J. Mol. Catal. B Enzym. 2003, 22, 369–376.
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the Preparation of Fish Protein Anti-Obesity Hydrolysates Using Response Surface Methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139.
- Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. Int. J. Mol. Sci. 2023, 24, 1529.
- Suwanangul, S.; Sangsawad, P.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Chittrakorn, S.; Ruttarattanamongkol, K. Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Protein Isolate and Its Hydrolysates Produced with Different Proteases. Maejo Int. J. Sci. Technol. 2021, 15, 48–60.
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae 2022, 8, 344.
- Ugusman, A.; Shahrin, S.A.S.; Azizan, N.H.; Pillai, S.B.; Krishnan, K.; Salamt, N.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Mokhtar, M.H. Role of Honey in Obesity Management: A Systematic Review. Front. Nutr. 2022, 9, 924097.
- Čolak, E.; Pap, D. The Role of Oxidative Stress in the Development of Obesity and Obesity-Related Metabolic Disorders. J. Med. Biochem. 2021, 40, 1–9.
- Savini, I.; Catani, M.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538.
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in Cardiovascular Diseases. Redox Biol. 2019, 23, 101119.
- Duryee, M.J.; Clemens, D.L.; Opperman, P.J.; Thiele, G.M.; Duryee, L.M.; Garvin, R.P.; Anderson, D.R. Malondialdehyde-Acetaldehyde Modified (MAA) Proteins Differentially Effect the Inflammatory Response in Macrophage, Endothelial Cells and Animal Models of Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 12948.
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable Oils Rich in Alpha Linolenic Acid Increment Hepatic N-3 LCPUFA, Modulating the Fatty Acid Metabolism and Antioxidant Response in Rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35.
- Yan, K.; Deng, X.; Zhai, X.; Zhou, M.; Jia, X.; Luo, L.; Niu, M.; Zhu, H.; Qiang, H.; Zhou, Y. P38 Mitogen-Activated Protein Kinase and Liver X Receptor-α Mediate the Leptin Effect on Sterol Regulatory Element Binding Protein-1c Expression in Hepatic Stellate Cells. Mol. Med. 2012, 18, 10–18.
- de Faria, A.P.; Ritter, A.M.V.; Gasparetti, C.S.; Corrêa, N.B.; Brunelli, V.; Almeida, A.; Pires, N.F.; Modolo, R.; Moreno Junior, H. A Proposed Inflammatory Score of Circulating Cytokines/Adipokines Associated with Resistant Hypertension, but Dependent on Obesity Parameters. Arq. Bras. Cardiol. 2019, 112, 383–389.
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The Role of Leptin and Ghrelin in the Regulation of Food Intake and Body Weight in Humans: A Review. Obes. Rev. 2007, 8, 21–34.
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia Is Required for the Development of Leptin Resistance. PLoS ONE 2010, 5, e11376.
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51.
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190.
- Othman, N.S.; Aminuddin, A.; Zainal Abidin, S.; Syafruddin, S.E.; Ahmad, M.F.; Mohd Mokhtar, N.; Kumar, J.; Hamid, A.A.; Ugusman, A. Profiling of Differentially Expressed MicroRNAs in Human Umbilical Vein Endothelial Cells Exposed to Hyperglycemia via RNA Sequencing. Life 2023, 13, 1296.
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of Edible Brown Algae Extracts for the Inhibition of Intestinal Carbohydrate Digestive Enzymes Involved in Glucose Release from the Diet. J. Nutr. Sci. 2021, 10, e5.
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692.
- You, Z.; Li, Y.; Zhang, K.; Zheng, X.; Wong, V.K.W.; Liu, W. Inhibitory Effect of Plant Essential Oils on α-Glucosidase. Food Sci. Biotechnol. 2022, 31, 1593–1602.
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between Phenolic Compounds, Amylolytic Enzymes and Starch: An Updated Overview. Curr. Opin. Food Sci. 2020, 31, 102–113.
- Taslimi, P.; Gulçin, İ. Antidiabetic Potential: In Vitro Inhibition Effects of Some Natural Phenolic Compounds on α-Glycosidase and α-Amylase Enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956.
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (in Vitro): A Comparative Study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170.
- TADERA, K.; MINAMI, Y.; TAKAMATSU, K.; MATSUOKA, T. Inhibition of ALPHA.-Glucosidase and ALPHA.-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153.
- Zhang, A.; Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Dipeptidyl Peptidase-IV Inhibitory Peptides from Sacha Inchi Meal. J. Sci. Food Agric. 2023, 103, 2926–2938.
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the Mechanism of Cell Death Resulting from Streptozotocin Challenge in Experimental Animals, Its Practical Use and Potential Risk to Humans. J. Diabetes Metab. Disord. 2013, 12, 60.
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A Practical Guide for Induction of Type-2 Diabetes in Rat: Incorporating a High-Fat Diet and Streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613.
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546.
- Zhu, B.T. Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells. Cells 2022, 11, 492.
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78.
- Mathias Akinlade, O.; Victor Owoyele, B.; Olufemi Soladoye, A. Streptozotocin-Induced Type 1 and 2 Diabetes in Rodents: A Model for Studying Diabetic Cardiac Autonomic Neuropathy. Afr. Health Sci. 2021, 21, 719–727.
- Rojanaverawong, W.; Wongmanee, N.; Hanchang, W. Sacha Inchi (Plukenetia volubilis L.) Oil Improves Hepatic Insulin Sensitivity and Glucose Metabolism through Insulin Signaling Pathway in a Rat Model of Type 2 Diabetes. Prev. Nutr. Food Sci. 2023, 28, 30–42.
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216.
- Jiang, S.; Young, J.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced Alterations in Hepatic Glucose and Lipid Metabolism: The Role of Type 1 and Type 2 Diabetes Mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611.
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin Regulation of Gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35.
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590.
- Yan, J.; Sheng, L.; Li, H. Akkermansia muciniphila: Is It the Holy Grail for Ameliorating Metabolic Diseases? Gut Microbes 2021, 13, 1984104.
- Bahar-Tokman, H.; Demirci, M.; Keskin, F.; Cagatay, P.; Taner, Z.; Ozturk-Bakar, Y.; Ozyazar, M.; Kiraz, N.; Kocazeybek, B. Firmicutes/Bacteroidetes Ratio in the Gut Microbiota and IL-1β, IL-6, IL-8, TLR2, TLR4, TLR5 Gene Expressions in Type 2 Diabetes. Clin. Lab. 2022, 68, 1903–1910.
- Fassatoui, M.; Lopez-Siles, M.; Díaz-Rizzolo, D.A.; Jmel, H.; Naouali, C.; Abdessalem, G.; Chikhaoui, A.; Nadal, B.; Jamoussi, H.; Abid, A.; et al. Gut Microbiota Imbalances in Tunisian Participants with Type 1 and Type 2 Diabetes Mellitus. Biosci. Rep. 2019, 39, BSR20182348.
- Demirci, M.; Bahar Tokman, H.; Taner, Z.; Keskin, F.E.; Çağatay, P.; Ozturk Bakar, Y.; Özyazar, M.; Kiraz, N.; Kocazeybek, B.S. Bacteroidetes and Firmicutes Levels in Gut Microbiota and Effects of Hosts TLR2/TLR4 Gene Expression Levels in Adult Type 1 Diabetes Patients in Istanbul, Turkey. J. Diabetes Complicat. 2020, 34, 107449.
- Lin, J.; Wen, J.; Xiao, N.; Cai, Y.T.; Xiao, J.; Dai, W.; Chen, J.P.; Zeng, K.W.; Liu, F.; Du, B.; et al. Anti-Diabetic and Gut Microbiota Modulation Effects of Sacha Inchi (Plukenetia volubilis L.) Leaf Extract in Streptozotocin-Induced Type 1 Diabetic Mice. J. Sci. Food Agric. 2022, 102, 4304–4312.
- Alayón, A.N.; Ortega Avila, J.G.; Echeverri Jiménez, I. Carbohydrate Metabolism and Gene Expression of Sirtuin 1 in Healthy Subjects after Sacha Inchi Oil Supplementation: A Randomized Trial. Food Funct. 2018, 9, 1570–1577.
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in Glucose and Lipid Metabolism. Oncotarget 2017, 8, 1845–1859.
- Zhou, S.; Tang, X.; Chen, H.-Z. Sirtuins and Insulin Resistance. Front. Endocrinol. 2018, 9, 748.
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, B.S. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141.
- Son, J.S.; Choi, S.; Lee, G.; Jeong, S.-M.; Kim, S.M.; Kim, K.; Yun, J.M.; Park, S.M. Blood Pressure Change from Normal to 2017 ACC/AHA Defined Stage 1 Hypertension and Cardiovascular Risk. J. Clin. Med. 2019, 8, 820.
- Losurdo, P.; Grillo, A.; Panizon, E.; Cappellari, G.G.; Fabris, B.; Bardelli, M.; Biolo, G.; Zanetti, M.; Carretta, R. Supplementation of Omega-3 Polyunsaturated Fatty Acids Prevents Increase in Arterial Stiffness After Experimental Menopause. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 114–120.
- Spector, A.A.; Kim, H.-Y. Discovery of Essential Fatty Acids. J. Lipid Res. 2015, 56, 11–21.
- Bentsen, H. Dietary Polyunsaturated Fatty Acids, Brain Function and Mental Health. Microb. Ecol. Health Dis. 2017, 28, 1281916.
- Wang, H.; Li, Q.; Zhu, Y.; Zhang, X. Omega-3 Polyunsaturated Fatty Acids: Versatile Roles in Blood Pressure Regulation. Antioxid. Redox Signal. 2021, 34, 800–810.
- Ferrari, R. RAAS Inhibition and Mortality in Hypertension. Glob. Cardiol. Sci. Pract. 2013, 2013, 34.
- Chirinos, R.; Pedreschi, R.; Campos, D. Enzyme-Assisted Hydrolysates from Sacha Inchi (Plukenetia Volubilis) Protein with in Vitro Antioxidant and Antihypertensive Properties. J. Food Process. Preserv. 2020, 44, e14969.
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P.V. Antihypertensive Effect of Caffeic Acid and Its Analogs through Dual Renin–Angiotensin–Aldosterone System Inhibition. Eur. J. Pharmacol. 2014, 730, 125–132.
- Blaustein, M.P.; Leenen, F.H.H.; Chen, L.; Golovina, V.A.; Hamlyn, J.M.; Pallone, T.L.; Van Huysse, J.W.; Zhang, J.; Wier, W.G. How NaCl Raises Blood Pressure: A New Paradigm for the Pathogenesis of Salt-Dependent Hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H1031–H1049.
- Obradovic, M.; Sudar-Milovanovic, E.; Gluvic, Z.; Banjac, K.; Rizzo, M.; Isenovic, E.R. The Na+/K+-ATPase: A Potential Therapeutic Target in Cardiometabolic Diseases. Front. Endocrinol. 2023, 14, 1150171.
- Li, P.; Cai, X.; Xiao, N.; Ma, X.; Zeng, L.; Zhang, L.-H.; Xie, L.; Du, B. Sacha Inchi (Plukenetia volubilis L.) Shell Extract Alleviates Hypertension in Association with the Regulation of Gut Microbiota. Food Funct. 2020, 11, 8051–8067.
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension—Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172.
- Lamb, F.S.; Choi, H.; Miller, M.R.; Stark, R.J. TNFα and Reactive Oxygen Signaling in Vascular Smooth Muscle Cells in Hypertension and Atherosclerosis. Am. J. Hypertens. 2020, 33, 902–913.
- Rybalkin, S.D.; Rybalkina, I.G.; Fei, R.; Hofmann, F.; Beavo, J.A. Regulation of CGMP-Specific Phosphodiesterase (PDE5) Phosphorylation in Smooth Muscle Cells. J. Biol. Chem. 2002, 277, 3310–3317.
- Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C.; Rochette, L. Iron, Oxidative Stress, and Redox Signaling in the Cardiovascular System. Mol. Nutr. Food Res. 2014, 58, 1721–1738.
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial Function and Dysfunction: Impact of Sodium-Glucose Cotransporter 2 Inhibitors. Pharmacol. Ther. 2021, 224, 107832.
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial Dysfunction, Endothelial Nitric Oxide Bioavailability, Tetrahydrobiopterin, and 5-Methyltetrahydrofolate in Cardiovascular Disease. Where Are We with Therapy? Microvasc. Res. 2018, 119, 7–12.
- Al Samarraie, A.; Pichette, M.; Rousseau, G. Role of the Gut Microbiome in the Development of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 5420.
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087.
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690.
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715.
- Yan, D.; Sun, Y.; Zhou, X.; Si, W.; Liu, J.; Li, M.; Wu, M. Regulatory Effect of Gut Microbes on Blood Pressure. Anim. Models Exp. Med. 2022, 5, 513–531.
This entry is offline, you can click here to edit this entry!
