Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Lung infections are one of the most common causes of death and morbidity worldwide. Both bacterial and viral lung infections cause a vast number of infections with varying severities. Extracellular vesicles (EVs) produced by different cells due to infection in the lung have the ability to modify the immune system, leading to either better immune response or worsening of the disease. It has been shown that both bacteria and viruses have the ability to produce their EVs and stimulate the immune system for that.
- extracellular vesicles
- exosomes
- lung infection
- bacterial
- host
1. Introduction
Extracellular vesicles (EVs) are eukaryotic- and prokaryotic-cell-derived heterogeneous populations of lipid-bilayer-membrane-enclosed structures, which are released in the extracellular space [1][2][3]. Eukaryotic-cell-derived EVs are of high importance in disease pathogens. The release of vesicles by cells into the extracellular matrix was first described as a method of discarding unneeded materials almost forty years ago [4][5]. However, it has been known that together with receptor ligands, signaling molecules, and hormones, EVs are necessary for cell–cell communication and interaction to achieve the coordination of almost all physiologic and metabolic processes [2]. Over the years, there have been multiple classifications for EVs [6][7][8][9][10]. Bacterial EVs, on the one hand, had their distinctive nomenclature: outer membrane vesicles (OMV) in gram-negative bacteria and extracellular vesicles or membrane vesicles (EVs or MVs) in gram-positive bacteria [11]. On the other hand, in eukaryotic cells, EV experts have now classified EVs into two main distinct groups:
Ectosomes (also termed microvesicles, microparticles, or oncosomes), range between 50–10,000 nm in diameter, are formed and released by the outward budding of the plasma membrane [12][13]. Particles of this group possess special markers, such as annexin A1 and ADP-ribosylation factor 6 (ARF6) [9][13]. Since the ectosomes are formed by the outward budding of the plasma membrane, they show a similar composition apart from that the lipid composition among the ectosomes membrane bilayer is evenly distributed compared to the asymmetrical distribution of the lipids in the two leaflets of the plasma membrane [14][15][16]. The protein content of the ectosome’s membrane bilayer and cargo is similar to the plasma membrane and the cytosolic proteins of the producing cell. However, due to the formation by budding, ectosomes lack proteins directly associated with organelles such as the nucleus, Golgi apparatus, and the endoplasmic reticulum, since these organelles do not contribute to the biogenesis of ectosomes [17][18]. Interestingly, however, mitochondrial content has been discovered within the ectosomes [19].
Exosomes 30–150 nm in diameter are produced through the endocytic pathway, where the endosomal membrane experiences an inward buddying that results in what is called intraluminal vesicles (ILVs) [1]. ILVs resemble the pre-released exosomes. Following the formation of ILVs, the endosome is now called a multivesicular endosome (MVE), which releases its content of exosomes to the pericellular matrix after fusion with the plasma membrane [20]. Although the available isolation methodologies and techniques permit the classification of EVs based on surface antigen, density, and size, these strategies cannot determine the site of biogenesis of these EVs, making the identification of the two groups (ectosomes and exosomes) challenging due to the overlap between their particles’ composition and size [21]. Like ectosomes, exosomes have several distinguishing markers, such as the endosomal complexes required for transport (ESCRT): Alix, flotillin, TSG101, and Ras-associated binding protein (Rab) 5b proteins and tetraspanin members CD63, CD9, CD81, and CD82 [7][22][23]. Additionally, exosomes carry the cytosolic proteins Rabs (Ras-associated binding proteins), which participate in promoting exosome docking and membrane fusion [24][25], and annexins, a protein family whose members are presumed to regulate the dynamics of the membrane cytoskeleton and membrane fusion events [25]. The exosomes also carry lipids and metabolites [26][27] as well as a nucleic acid cargo, which is included among the functionally active exosomes when fused with the recipient cells. This may involve a range of non-coding RNAs, like miRNA; long non-coding RNA (lncRNA); fragments of tRNA; structural RNAs; small interfering RNAs; small RNA transcripts; and RNA-protein complexes. In addition to the different RNA species, exosome cargo contains DNA that could represent the whole genome and the genomic mutations [28][29][30][31] as well as mitochondrial DNA [32][33] (Figure 1).
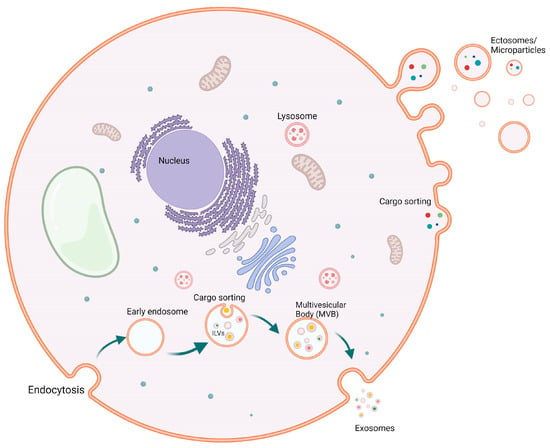
Figure 1. Biogenesis of extracellular vesicles (EVs). Biogenesis of both exosomes (50–150 mm) and ectosomes (50–10,000 mm) from healthy cells. Exosomes are budding from early endosomes after cargo sorting, where multivesicular bodies are formed, which then give rise to exosomes. On the other hand, ectosomes or microparticles are bigger particles that are budded through the cell membrane carrying various cargos.
Moreover, other EV subtypes, such as apoptotic bodies (50–5000), that are released by apoptotic cells have been described. They carry phosphatidylserine as a unique marker [34]. Furthermore, migrasomes 500–3000 nm, have been recently characterized as a type of EV formed from the retraction fibers and released to the periplasmic matrix during cell migration. They carry tetraspanin 4 (TSPAN4) as a marker to their subtype [35]. More newly identified subtypes of EVs have been reported: exophers 1000–10,000 nm, possess markers such as microtubule-associated protein 1A/1B-light chain 3 (LC3), and mitochondrial import receptor subunit 20 (TOM20) [36]. Expophers are yet to be studied in humans. Lastly, exomeres < 50 nm, approximately 35 nm, have been recently described as non-membranous particles [37]. These particles, with the other EVs and particles derived from tumors, have been reported to induce the re-metastatic niches in the formation and pathological effects of organs. Furthermore, they can promote inflammation and downregulation of lipid catabolism in non-tumor livers [38].
2. Extracellular Vesicles in Bacterial Lung Infections
Host EVs that are released by the host have been thoroughly investigated in various bacterial lung infections. In tuberculosis, the isolated EVs from the cells infected by Mycobacterium tuberculosis (Mtb) promote the recruitment and activation of immune cells. Thus, they contribute to the innate immune response. Furthermore, EVs have been suggested to be significant for antigen presentation in Mtb-infected mice, where the infected Siglec-1 knockout mice showed higher apparency in the local spread of bacteria compared to the wild-type mice despite the same bacterial load, suggesting that Siglic-1 is important to inducing antigen presentation, which is mediated by EVs [39]. Immunologically, EVs from Mtb-infected macrophages were also able to activate the endothelial cells, promoting cell migration through the cell monolayer. This also includes the upregulation of genes such as ccl2, vcam 1, and cxc1, cytokine–cytokine receptor interaction, and increased levels of chemokines and adhesion molecules, e.g., chemokine (C-C motif) ligand 2 (CCL2) and VCAM1 [40]. It has been found that exosomes derived from THP-1 cells infected by Mycobacterium bovis (BCG) can initiate a proinflammatory response in mice with a robust production of IL-12p40 and tumor necrosis factor alpha (TNF-α) in the lung [41]. On the other hand, evaluating the effects of serum EVs from Mtb-infected patients on the viability of THP-1 monocytes and the human peripheral blood mononuclear cells (PBMCs) showed that infecting both cell types with patient serum EVs, led to an increased death rate [42]. Moreover, a study found that treating Mtb-infected macrophages with EVs produced by Mtb-infected neutrophils increases the production of superoxide anion and autophagy in macrophages leading to higher bacterial killing, as higher levels of proinflammatory cytokines (TNF-α, IL-6, and IL-10) were observed in macrophages stimulated with EVs isolated from Mtb-infected neutrophils [43]. Additionally, it was demonstrated that Mtb RNA is transferred in the macrophage-derived EVs through a SecA2-dependent pathway. These EVs cause the induction of IFN response in macrophages, which is based on the foreign-RNA-sensing pathway retinoic-acid-inducible gene I (RIG-I)/MAVS/TBK1/IRF3 [44]. The activation of this pathway is needed for the EVs produced by Mtb-infected macrophages to generate Mtb replication restriction in the surrounding macrophages [44]. Another study reported that EVs isolated from infected J774A.1 macrophages were larger than spontaneously released EVs (S-EV) from the same uninfected cell line. In addition, Mtb-EV was observed to contain M. tuberculosis antigens. The study also showed that S-EV reduced the bacterial load and production level of both MCP-1 and TNF-α in M. tuberculosis-infected macrophages. Moreover, treating mice infected with M. tuberculosis with both S-EV and Mtb-EV led to a significant reduction of bacterial load in the lungs, but no effect was recorded regarding the survival rate or the lung pneumonic area [45].
On the aspect of adaptive immune response, its activation can also be improved by EVs from Mtb-infected cells. It has been shown that the EVs from macrophages infected by Mycobacterium tuberculosis and Mycobacterium bovis (BCG) can activate the T cells by presenting peptide-MHC-II complexes [46]. Treating mice with EVs from BCG-infected macrophages leads to antigen-specific activation of CD4+ and CD8+ T cells in the spleen, lung, and mediastinal nodes [47].
On the contrary, immune suppression was observed upon treatment with EVs from mycobacterial-infected cells. The expression of macrophages MHC-II and CD4 in response to IFN-γ was partially inhibited upon treatment with EVs produced by Mtb-infected macrophages, which is dependent on TLR2 [48]. In addition, a similar but stronger inhibition of CD4+ T cell activation was observed compared to the inhibition caused by lipoarabinomannan (LAM), a virulence factor associated with Mtb. This led to a reduction of IL-2 production and T-cell proliferation [49].
Streptococcus pneumoniae is another species that causes bacterial lung infection called pneumococcal pneumonia. Pneumolysin is a pore-forming toxin produced by S. pneumoniae and a main contributor to the inflammatory processes implicated in the aforementioned infection [50]. It has been found that the EVs produced by the neutrophils subjected to pneumolysin can cause direct activation of platelets. This included a significant increase in the percentage of platelets expressing CD62P (a P-selectin present in megakaryocytes) and a higher surface expression of CD62P compared to the platelets incubated with the EVs collected from untreated neutrophils [50]. Another finding reported that naïve platelets incubated with EVs produced by neutrophils treated with pneumolysin showed a higher percentage of cells producing CD62P and increased level of surface expression of the same protein with respect to naïve platelets incubated with EVs isolated from non-pneumolysin-challenged neutrophils [50]. An additional study showed that microvesicles isolated from lung epithelial cells stimulated with pneumolysin were able to impair the ROS production in neutrophils, thus suppressing these neutrophils’ defensive response [51].
Staphylococcus aureus can cause real life-threatening infections in humans. In some cases, it can lead to sepsis and both pneumonia and bone and joint infections. It has been shown that EVs produced by neutrophils treated with S. aureus (termed bEVs) can be associated with the exogenously added bacteria causing aggregation [52]. In accordance with that, another study revealed that bEVs were able to induce the proinflammatory response in macrophages exhibited by producing both IL-6 and IL-1β in a dose-dependent manner [53].
Pseudomonas aeruginosa is a pathogen that has been reported to form antibiotic-resistant biofilms. Interestingly, a study demonstrated that miRNA (let-7b-5b) transferred by EVs secreted by the airway epithelial cells can act as an RNA interference to reduce the ability of P. aeruginosa to form biofilms and increase the sensitivity of this pathogen to beta-lactam antibiotic aztreonam [54].
Legionella peneumophila is a bacterial agent that causes severe pneumonia in humans. EVs collected from supernatants of L. pneumophila-infected THP-1 were used to infect both THP-1 and A549 healthy cells. The results demonstrated a higher response in THP-1 cells in CXCL8, TNF-α, IL-1β, and MCP-1 expression compared to A549 cells [55] (Figure 2).
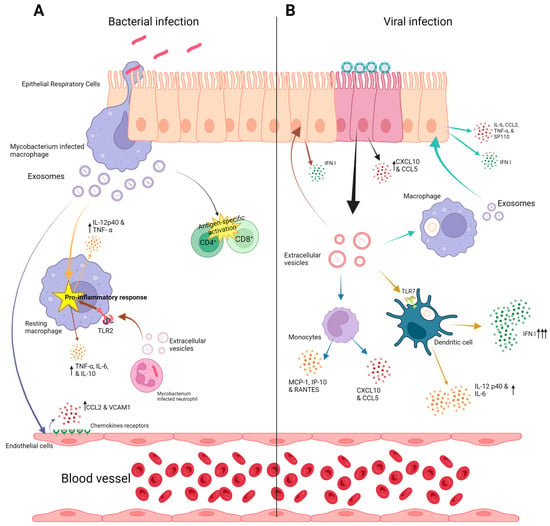
Figure 2. Exosome function in lung infections (A); bacterial lung infection: In bacterial lung infections, epithelial cells, macrophages, and endothelial cells play an important role in pathogenesis through their exosomal cargo. Various cytokines and chemokine production due to exosome effects are shown. (B) Viral lung infection: The contribution of exosomal cargo from macrophages, epithelial cells, monocytes, and dendritic cells is depicted. Various cytokines and chemokine production due to exosome effects are shown. Upward arrows indicate increased production of various chemokines and cytokines.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242216139
References
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 175.
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727.
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948.
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685.
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Thery, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565.
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593.
- Bose, S.; Aggarwal, S.; Singh, D.V.; Acharya, N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microb. Cell 2020, 7, 312–322.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232.
- Hugel, B.; Martinez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27.
- Scott, S.; Pendlebury, S.A.; Green, C. Lipid organization in erythrocyte membrane microvesicles. Biochem. J. 1984, 224, 285–290.
- Kastelowitz, N.; Yin, H. Exosomes and microvesicles: Identification and targeting by particle size and lipid chemical probes. Chembiochem 2014, 15, 923–928.
- Sinha, A.; Ignatchenko, V.; Ignatchenko, A.; Mejia-Guerrero, S.; Kislinger, T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem. Biophys. Res. Commun. 2014, 445, 694–701.
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385.
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pepin, G.; Germain, M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971.
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668.
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948.
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031.
- Schorey, J.S.; Bhatnagar, S. Exosome function: From tumor immunology to pathogen biology. Traffic 2008, 9, 871–881.
- Rani, S.; O’Brien, K.; Kelleher, F.C.; Corcoran, C.; Germano, S.; Radomski, M.W.; Crown, J.; O’Driscoll, L. Isolation of exosomes for subsequent mRNA, MicroRNA, and protein profiling. Methods Mol. Biol. 2011, 784, 181–195.
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360.
- Qu, X.; Li, Q.; Yang, J.; Zhao, H.; Wang, F.; Zhang, F.; Zhang, S.; Zhang, H.; Wang, R.; Wang, Q.; et al. Double-Stranded DNA in Exosomes of Malignant Pleural Effusions as a Novel DNA Source for EGFR Mutation Detection in Lung Adenocarcinoma. Front. Oncol. 2019, 9, 931.
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915.
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769.
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 2018, 7, 1505403.
- Cai, J.; Han, Y.; Ren, H.; Chen, C.; He, D.; Zhou, L.; Eisner, G.M.; Asico, L.D.; Jose, P.A.; Zeng, C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J. Mol. Cell Biol. 2013, 5, 227–238.
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernandez-Delgado, I.; Latorre-Pellicer, A.; Acin-Perez, R.; Martin-Cofreces, N.B.; Jaso-Tamame, A.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658.
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88.
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015, 25, 24–38.
- Nicolas-Avila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martinez, L.; Sanchez-Diaz, M.; Diaz-Garcia, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109 e123.
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343.
- Wang, G.; Li, J.; Bojmar, L.; Chen, H.; Li, Z.; Tobias, G.C.; Hu, M.; Homan, E.A.; Lucotti, S.; Zhao, F.; et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 2023, 618, 374–382.
- Benet, S.; Galvez, C.; Drobniewski, F.; Kontsevaya, I.; Arias, L.; Monguio-Tortajada, M.; Erkizia, I.; Urrea, V.; Ong, R.Y.; Luquin, M.; et al. Dissemination of Mycobacterium tuberculosis is associated to a SIGLEC1 null variant that limits antigen exchange via trafficking extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12046.
- Li, L.; Cheng, Y.; Emrich, S.; Schorey, J. Activation of endothelial cells by extracellular vesicles derived from Mycobacterium tuberculosis infected macrophages or mice. PLoS ONE 2018, 13, e0198337.
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244.
- Javadi, A.; Shamaei, M.; Tabarsi, P.; Nomani, M.; Varahram, M.; Kazemi, B. Extracellular vesicles from serum samples of mycobacteria patients induced cell death of THP-1 monocyte and PBMC. BMC Pulm. Med. 2022, 22, 57.
- Alvarez-Jimenez, V.D.; Leyva-Paredes, K.; Garcia-Martinez, M.; Vazquez-Flores, L.; Garcia-Paredes, V.G.; Campillo-Navarro, M.; Romo-Cruz, I.; Rosales-Garcia, V.H.; Castaneda-Casimiro, J.; Gonzalez-Pozos, S.; et al. Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival. Front. Immunol. 2018, 9, 272.
- Cheng, Y.; Schorey, J.S. Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep. 2019, 20, e46613.
- Garcia-Martinez, M.; Vazquez-Flores, L.; Alvarez-Jimenez, V.D.; Castaneda-Casimiro, J.; Ibanez-Hernandez, M.; Sanchez-Torres, L.E.; Barrios-Payan, J.; Mata-Espinosa, D.; Estrada-Parra, S.; Chacon-Salinas, R.; et al. Extracellular vesicles released by J774A.1 macrophages reduce the bacterial load in macrophages and in an experimental mouse model of tuberculosis. Int. J. Nanomed. 2019, 14, 6707–6719.
- Ramachandra, L.; Qu, Y.; Wang, Y.; Lewis, C.J.; Cobb, B.A.; Takatsu, K.; Boom, W.H.; Dubyak, G.R.; Harding, C.V. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect. Immun. 2010, 78, 5116–5125.
- Giri, P.K.; Schorey, J.S. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 2008, 3, e2461.
- Singh, P.P.; LeMaire, C.; Tan, J.C.; Zeng, E.; Schorey, J.S. Exosomes released from M. tuberculosis infected cells can suppress IFN-gamma mediated activation of naive macrophages. PLoS ONE 2011, 6, e18564.
- Athman, J.J.; Sande, O.J.; Groft, S.G.; Reba, S.M.; Nagy, N.; Wearsch, P.A.; Richardson, E.T.; Rojas, R.; Boom, W.H.; Shukla, S.; et al. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. 2017, 198, 2028–2037.
- Letsiou, E.; Teixeira Alves, L.G.; Felten, M.; Mitchell, T.J.; Muller-Redetzky, H.C.; Dudek, S.M.; Witzenrath, M. Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure. Cells 2021, 10, 3581.
- Letsiou, E.; Teixeira Alves, L.G.; Fatykhova, D.; Felten, M.; Mitchell, T.J.; Muller-Redetzky, H.C.; Hocke, A.C.; Witzenrath, M. Microvesicles released from pneumolysin-stimulated lung epithelial cells carry mitochondrial cargo and suppress neutrophil oxidative burst. Sci. Rep. 2021, 11, 9529.
- Herrmann, I.K.; Bertazzo, S.; O’Callaghan, D.J.; Schlegel, A.A.; Kallepitis, C.; Antcliffe, D.B.; Gordon, A.C.; Stevens, M.M. Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale 2015, 7, 13511–13520.
- Allen, E.R.; Lempke, S.L.; Miller, M.M.; Bush, D.M.; Braswell, B.G.; Estes, C.L.; Benedict, E.L.; Mahon, A.R.; Sabo, S.L.; Greenlee-Wacker, M.C. Effect of extracellular vesicles from S. aureus-challenged human neutrophils on macrophages. J. Leukoc. Biol. 2020, 108, 1841–1850.
- Koeppen, K.; Nymon, A.; Barnaby, R.; Bashor, L.; Li, Z.; Hampton, T.H.; Liefeld, A.E.; Kolling, F.W.; LaCroix, I.S.; Gerber, S.A.; et al. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2105370118.
- Jung, A.L.; Herkt, C.E.; Schulz, C.; Bolte, K.; Seidel, K.; Scheller, N.; Sittka-Stark, A.; Bertrams, W.; Schmeck, B. Legionella pneumophila infection activates bystander cells differentially by bacterial and host cell vesicles. Sci. Rep. 2017, 7, 6301.
This entry is offline, you can click here to edit this entry!
