Metal selenide materials have attracted attention due to their wide application prospects. In this family of materials, FeSe is particularly studied since it is both a semiconductor used in solar cells and a superconductor with a critical transition temperature, Tc, of 8 K. For any envisaged application, the possibility of preparing large-area FeSe thin films at low cost is extremely appealing, and one possible technique suitable for this purpose is electrodeposition. Several groups have reported successful electrodeposition of FeSe, but the investigated systems are different in many aspects, and the results are difficult to compare.
- iron selenide
- electrodeposition
- thin films
1. Introduction
2. FeSe Structure
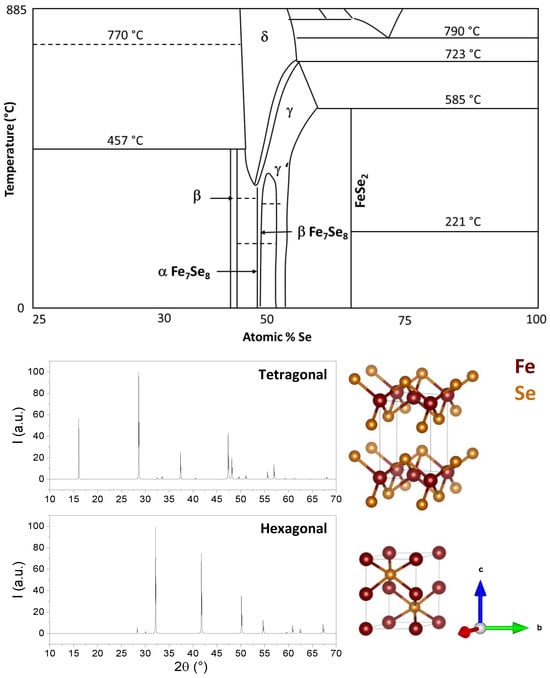
3. Thermodynamic Analysis
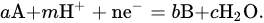
| a, b, c, n, m | Stoichiometric Number | |
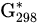 |
Standard Gibbs free energy at 298 K | kJ mol−1 |
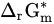 |
Standard molar reactive Gibbs free energy | kJ mol−1 |
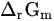 |
Molar reactive Gibbs free energy | kJ mol−1 |
| R | Molar gas constant | 8.314 J K−1 mol−1 at 298 K |
| T | Temperature | K |
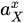 |
Activity of substance X | |
| F | Faraday’s constant | 96,485 C mol−1 |
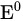 |
Standard electrode potential | V |
| E | Electrode Potential | V |
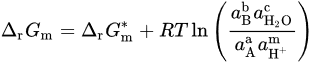
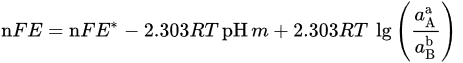
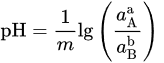
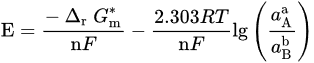
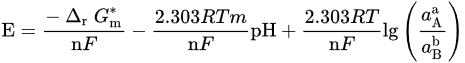
| Substance | 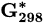 (kJ/mol) (kJ/mol) |
Substance | 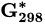 (kJ/mol) (kJ/mol) |
|---|---|---|---|
| (kJ/mol) | |||
| FeSe | −87.533 | Fe | −8.31 |
| Fe2+ | −35.585 | Fe3+ | 64.332 |
| Se | −12.592 | HSe− | −14.035 |
| H2Se (g) | −35.950 | SeO32− | −525.577 |
| H2SeO3 | −569.233 | HSeO3− | −560.894 |
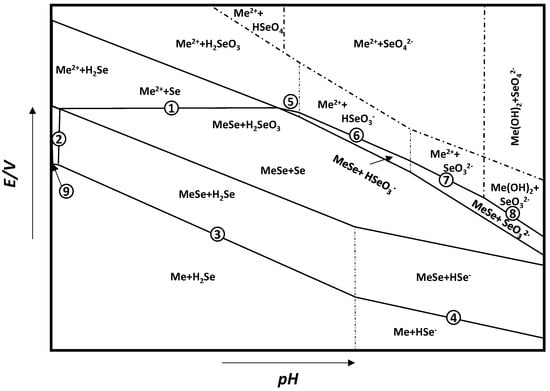
| Potential–pH formulas for FeSe0.96 | |||
| Fe2+ + 2e− = Fe | E = −0.5985 | ||
| Fe2+ + 0.96Se + 2e− = FeSe0.96 | E = −0.249 | ||
| FeSe0.96 + 1.92H+ + 1.92e− = Fe + 0.96H2Se | E = −0.278 − 0.059pH | ||
| FeSe0.96 + 0.96H+ + 1.92e− = Fe + 0.96HSe− | E = −0.424 − 0.030pH | ||
| Fe2+ + 0.96SeO32− +5.76H+ + 5.84e− = FeSe0.96 + 2.88H2O | E = 0.430 − 0.058pH | ||
4. Electrodeposition of FeSe
-
The electrolyte: i.e., the precursors and the solution. Different precursors give different results even if the element to be deposited is in the same oxidation state. Solubility, solvation effects, and other phenomena indirectly influence the reactions in the solution/on the electrode.
-
Electrode materials, working electrode potential (with respect to the reference electrode); even if the working electrode (substrate) is non-reacting, different materials will give different working potentials and, therefore, different results.
-
The pH of the solution influences the stability of the electrodes, the precursor salts, the conductivity of the solution, et cetera.
-
Additives/complexing agents: used to increase the solubility of precursors, they influence the adsorption of metal ions at the substrate surface, film nucleation, and growth.
-
The operation temperature (usually between room temperature and T < 100 °C)
This entry is adapted from the peer-reviewed paper 10.3390/coatings13111905
References
- Zhang, K.; Hu, Z.; Liu, X.; Tao, Z.; Chen, J. FeSe2 microspheres as a high-performance anode material for Na-ion batteries. Adv. Mater. 2015, 27, 3305–3309.
- Yoo, H.; Lee, G.H.; Kim, D.W. FeSe hollow spheroids as electrocatalysts for high-rate Li–O2 battery cathodes. J. Alloys Compd. 2021, 856, 158269.
- Wang, W.; Pan, X.; Liu, W.; Zhang, B.; Chen, H.; Fang, X.; Yao, J.; Dai, S. FeSe2 films with controllable morphologies as efficient counter electrodes for dye-sensitized solar cells. Chem. Commun. 2014, 50, 2618–2620.
- Kwon, J.; Jun, S.W.; Choi, S.I.; Mao, X.; Kim, J.; Koh, E.K.; Kim, Y.H.; Kim, S.K.; Hwang, D.Y.; Kim, C.S.; et al. FeSe quantum dots for in vivo multiphoton biomedical imaging. Sci. Adv. 2019, 5, eaay0044.
- Chanda, D.; Tufa, R.A.; Birdja, Y.Y.; Basu, S.; Liu, S. Hydrothermally/electrochemically decorated FeSe on Ni-foam electrode: An efficient bifunctional electrocatalysts for overall water splitting in an alkaline medium. Int. J. Hydrogen Energy 2020, 45, 27182–27192.
- Majhi, K.C.; Yadav, M. Transition metal chalcogenides based nanocomposites as efficient electrocatalyst for hydrogen evolution reaction over the entire pH range. Int. J. Hydrogen Energy 2020, 45, 24219–24231.
- Cheng, Z.; Gao, M.; Sun, L.; Zheng, D.; Xu, H.; Kong, L.; Gao, C.; Yu, H.; Lin, J. FeSe/FeSe2 Heterostructure as a Low-Cost and High-Performance Electrocatalyst for Oxygen Evolution Reaction. ChemElectroChem 2022, 9, e202200399.
- Hou, B.; Benito-alifonso, D.; Webster, R.F.; Cherns, D.; Galan, M.C.; Fermín, D.J. Synthetic mechanism studies of iron selenides: An emerging class of materials for electrocatalysis. Catalysts 2021, 11, 681.
- Iida, K.; Hänisch, J.; Tarantini, C. Fe-based superconducting thin films on metallic substrates: Growth, characteristics, and relevant properties. Appl. Phys. Rev. 2018, 5, 031304.
- Sakoda, M.; Iida, K.; Naito, M. Recent progress in thin-film growth of Fe-based superconductors: Superior superconductivity achieved by thin films. Supercond. Sci. Technol. 2018, 31, 093001.
- Piperno, L.; Vannozzi, A.; Augieri, A.; Masi, A.; Mancini, A.; Rufoloni, A.; Celentano, G.; Braccini, V.; Cialone, M.; Iebole, M.; et al. High-performance Fe(Se,Te) films on chemical CeO2-based buffer layers. Sci. Rep. 2023, 13, 569.
- Vannozzi, A.; Prili, S.; Sylva, G.; Masi, A.; Armenio, A.A.; Mancini, A.; Pinto, V.; Rufoloni, A.; Piperno, L.; Augieri, A.; et al. Epitaxial Zr-doped CeO2 films by chemical solution deposition as buffer layers for Fe(Se,Te) film growth. Supercond. Sci. Technol. 2020, 33, 9.
- Piperno, L.; Vannozzi, A.; Augieri, A.; Pinto, V.; Armenio, A.A.; Rizzo, F.; Mancini, A.; Rufoloni, A.; Celentano, G.; Braccini, V.; et al. Chemical CeO2-based buffer layers for Fe(Se,Te) films. IEEE Trans. Appl. Supercond. 2022, 32, 1–5.
- Alesini, D.; Braggio, C.; Carugno, G.; Crescini, N.; D’Agostino, D.; Di Gioacchino, D.; Di Vora, R.; Falferi, P.; Gallo, S.; Gambardella, U.; et al. Galactic axions search with a superconducting resonant cavity. Phys. Rev. D 2019, 99, 101101.
- Hussain, R.A.; Badshah, A.; Lal, B. Fabrication, characterization and applications of iron selenide. J. Solid State Chem. 2016, 243, 179–189.
- Pesko, E.; Zukowska, G.; Zero, E.; Krzton-Maziopa, A. Electrocrystallization of nanostructured iron-selenide films for potential application in dye sensitized solar cells. Thin Solid Film. 2020, 709, 138121.
- Demura, S.; Ozaki, T.; Okazaki, H.; Mizuguchi, Y.; Kawasaki, Y.; Deguchi, K.; Watanabe, T.; Hara, H.; Yamaguchi, T.; Takeya, H.; et al. Electrochemical synthesis of iron-based superconductor FeSe films. J. Phys. Soc. Jpn. 2012, 81, 043702.
- Demura, S.; Tanaka, M.; Yamashita, A.; Denholme, S.J.; Okazaki, H.; Fujioka, M.; Yamaguchi, T.; Takeya, H.; Iida, K.; Holzapfel, B.; et al. Electrochemical deposition of FeSe on RABiTS tapes. J. Phys. Soc. Jpn. 2016, 85, 015001.
- Demura, S.; Okazaki, H.; Ozaki, T.; Hara, H.; Kawasaki, Y.; Deguchi, K.; Watanabe, T.; Denholme, S.J.; Mizuguchi, Y.; Yamaguchi, T.; et al. Electrodeposition as a new route to synthesize superconducting FeSe. Solid State Commun. 2013, 154, 40–42.
- Okamoto, H. The fese (ironselenium) system. J. Phase Equilibria 1991, 12, 383–389.
- Sines, I.T.; Schaak, R.E. Phase-selective chemical extraction of selenium and sulfur from nanoscale metal chalcogenides: A general strategy for synthesis, purification, and phase targeting. J. Am. Chem. Soc. 2011, 133, 1294–1297.
- Amcoff; Ericsson, T.; Gismelseed, A. An X-ray, Mössbauer and magnetization investigation of hexagonal FeSe. Z. Krist. New Cryst. Struct. 1994, 209, 197–205.
- Haindl, S. Iron-Based Superconducting Thin Films; Springer International Publishing: Cham, Switzerland, 2021.
- Hsu, F.C.; Luo, J.Y.; Yeh, K.W.; Chen, T.K.; Huang, T.W.; Wu, P.M.; Lee, Y.C.; Huang, Y.L.; Chu, Y.Y.; Yan, D.C.; et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl. Acad. Sci. USA 2008, 105, 14262–14264.
- Margadonna, S.; Takabayashi, Y.; McDonald, M.T.; Kasperkiewicz, K.; Mizuguchi, Y.; Takano, Y.; Fitch, A.N.; Suard, E.; Prassides, K. Crystal structure of the new FeSe1−x superconductor. Chem. Commun. 2008, 43, 5607–5609.
- Kumar, R.S.; Zhang, Y.; Sinogeikin, S.; Xiao, Y.; Kumar, S.; Chow, P.; Cornelius, A.L.; Chen, C. Crystal and electronic structure of FeSe at high pressure and low temperature. J. Phys. Chem. B 2010, 114, 12597–12606.
- Ubale, A.U.; Sakhare, Y.S.; Bhute, M.V.; Belkhedkar, M.R.; Singh, A. Size-dependent structural, electrical and optical properties of nanostructured iron selenide thin films deposited by Chemical Bath Deposition Method. Solid State Sci. 2013, 16, 134–142.
- Wang, X.; Li, H.; Huang, Y.; Dong, Z.; Zhong, C.; Liu, J. Tuning tetrahedral structure and electronic properties of FeSe films through strain engineering. J. Phys. Chem. Solids 2020, 145, 109541.
- Hara, Y.; Takase, K.; Yamasaki, A.; Sato, H.; Miyakawa, N.; Umeyama, N.; Ikeda, S.I. Structural and physical properties of FeSe crystals fabricated by the chemical vapor transport method. Phys. C Supercond. Appl. 2010, 470, S313–S314.
- Guterding, D.; Jeschke, H.O.; Valentí, R. Basic electronic properties of iron selenide under variation of structural parameters. Phys. Rev. B 2017, 96, 125107.
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288.
- Minakshi, M.; Singh, P.; Issa, T.B.; Thurgate, S.; De Marco, R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery: Part III. Electrochemical behavior of γ-MnO2 in aqueous lithium hydroxide electrolyte. J. Power Sources 2006, 153, 165–169.
- Lai, Y.; Han, C.; Yan, C.; Liu, F.; Li, J.; Liu, Y. Thermodynamic analysis on metal selenides electrodeposition. J. Alloys Compd. 2013, 557, 40–46.
- Bouroushian, M. Electrochemistry of Metal Chalcogenides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010.
- Ray, A. Electrodeposition of Thin Films for Low-cost Solar Cells. In Electroplating of Nanostructures; IntechOpen: London, UK, 2015.
- Saloniemi, H. Electrodeposition of PbS, PbSe and PbTe Thin Films; VTT Publications: Espoo, Finland, 2000; pp. 2–82.
- Gao, Z.; Qi, J.; Chen, M.; Zhang, W.; Cao, R. An Electrodeposited NiSe for Electrocatalytic Hydrogen and Oxygen Evolution Reactions in Alkaline Solution. Electrochim. Acta 2017, 224, 412–418.
- Dhanasekaran, V.; Mahalingam, T.; Rhee, J.K.; Chu, J.P. Structural and optical properties of electrosynthesized ZnSe thin films. Optik 2013, 124, 255–260.
- Kowalik, R.; Kazimierczak, H.; Zabiński, P. Electrodeposition of cadmium selenide. Mater. Sci. Semicond. Process 2016, 50, 43–48.
- Kröger, F.A. Cathodic Deposition and Characterization of Metallic or Semiconducting Binary Alloys or Compounds. J. Electrochem. Soc. 1978, 125, 2028–2034.
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; American Association for the Advancement of Science (AAAS): Washington, DC, USA, 1966.
- Cook, W.G.; Olive, R.P. Pourbaix diagrams for the iron-water system extended to high-subcritical and low-supercritical conditions. Corros. Sci. 2012, 55, 326–331.
- Chen, P.Y.; Hu, S.F.; Liu, R.S.; Huang, C.Y. Electrodeposition of nano-dimensioned FeSe. Thin Solid Film. 2011, 519, 8397–8400.
- Zeynalova, A.O.; Javadova, S.P.; Majidzade, V.A.; Aliyev, A.S. Electrochemical Synthesis of Iron Monoselenide Thin Films. Chem. Probl. 2021, 19, 262–271.
- Aricò, A.S.; Antonucci, V.; Antonucci, P.L.; Cocke, D.L.; Giordano, N. A voltammetric study of the electrodeposition chemistry in the FeS system. Electrochim. Acta. 1991, 36, 581–590.
- Asakai, T.; Hioki, A. Evaluation of the Stability of Iron(II) Solutions by Precise Coulometric Titration with Electrogenerated Cerium(IV). Anal. Sci. 2012, 28, 601–606.
- Pawar, S.M.; Moholkar, A.V.; Suryavanshi, U.B.; Rajpure, K.Y.; Bhosale, C.H. Electrosynthesis and characterization of iron selenide thin films. Sol. Energy Mater. Sol. Cells 2007, 91, 560–565.
- Saji, V.S.; Lee, C.W. Selenium electrochemistry. RSC Adv. 2013, 3, 10058–10077.
