Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The majority of ethical and welfare approaches in animal research have primarily focused on vertebrates. Echinoderms are a phylum of marine invertebrates. They are often key, long-living species that shape and maintain the status of many marine ecosystems, inhabiting a wide range of ecological niches from the abyssal depths of the oceans to the intertidal zone. Progress has been made toward developing an ethical framework for invertebrates, such as crustaceans, echinoderms, and cephalopods.
- echinoderm welfare
- responsibility
- invertebrates
- echinoderm sentience
1. Phylum Echinodermata
Echinoderms are a phylum of marine invertebrates. They are often key, long-living species that shape and maintain the status of many marine ecosystems, inhabiting a wide range of ecological niches from the abyssal depths of the oceans to the intertidal zone. Echinoderms, distinguished by their pentamerous radial body arrangement, form a monophyletic group with hemichordates, also known as acorn worms. These organisms exhibit significant diversity and widespread distribution across a variety of marine habitats, playing critical ecological roles in each setting [1]. The fundamental characteristics of this phylum have exhibited remarkable consistency since the Ordovician epoch (approximately 495–440 million years ago) [2], with an approximate enumeration of 7000 species [3].
The extant echinoderm assemblage includes approximately 7000 species divided into five distinct taxonomic groups: Asteroidea, which includes starfishes; Echinoidea, which includes sea urchins, sand dollars, and sea biscuits; Crinoidea, which includes sea lilies and feather stars; Ophiuroidea, which includes basket stars and brittle stars; and Holothuroidea, which includes sea cucumbers. This collective lineage has an exceptionally notable fossil record dating back to the Cambrian period, providing a solid foundation for comparative molecular studies spanning a wide range of meticulously documented divergence intervals [2][3] (Figure 1).

Figure 1. Example of classes of echinoderms: (A) Cycethra verrucosa, Asteroidea from South Atlantic; (B) Arbacia dufresnii, Echinoidea from South Atlantic; (C) Ctenantedon kinziei, Crinoidea from the Caribbean; (D) Ophiocoma echinata, Ophiuroidea from the Caribbean; (E) Pearsonothuria graeffei, Holothuroidea from the Indo-Pacific.
Echinoderms have a number of distinguishing characteristics that set them apart from their zoological counterparts. Notably, among these characteristics is a hydraulic water vascular system that is intricately linked with a distinct arrangement of a calcium carbonate endoskeleton known as stereom. Most echinoderms’ developmental trajectories begin with larval stages, where intricate metamorphic processes culminate in the eventual manifestation of the adult form. These organisms exhibit a diverse range of life history traits, most notably sexual reproduction, though asexual reproduction is also observed. Echinoderm larvae are primitive, free-living, and planktonic in nature, with a wide range of morphology and functional characteristics that occasionally resemble those observed in hemichordate larvae. Following the metamorphic stage, the majority of mature echinoderms adopt a benthic lifestyle with radial symmetry and a typical pentameric structural composition. The organisms’ internal architecture is intricately organized, with a reliance on calcium carbonate ossicles reinforced by a complex network of collagenous ligaments. Notably, any skeletal structures found in larvae adopt intricate rod-like configurations with distinct origins in Ophiuroidea and Echinoidea, whereas such features are absent in the larvae of the remaining three echinoderm classes. Echinoderms have distinct pentaradial symmetry in their adult forms. Nonetheless, their developmental origins can be traced back to bilaterally symmetrical larvae, a shared feature of the deuterostome clade (shown in Figure 1A). These distinguishing characteristics place echinoderms within an intriguing evolutionary framework, identifying them as invertebrate deuterostomes inextricably linked to vertebrate organisms [4].
Echinoderm species exhibit a variety of developmental strategies, ranging from direct development from a fertilized egg to an adult to indirect development, in which adults emerge from the metamorphosis of a larva with no relation to the adult. The cell structure and count of indirect developing species’ long-living, feeding, bilaterally symmetrical larvae are very simple. There are numerous intermediate developmental stages, including facultative larval feeding and non-feeding larvae. Indirect development is primitive in echinoderms, and all five surviving classes, as well as the sister phylum Hemichordata, have dipleurula-type larvae [5].
Crinoids (Crinoidea) are sessile or free-living benthic animals with microphagous filter-feeding habits. Some comatulids feed during the day, while others feed by extending the tips of their arms or moving only at night to avoid predators. Some forms prefer and actively seek out areas with flowing water for feeding (rheophilic), while others do not (rheophobic). They can be found in a variety of habitats, including as attachments to substrates via their arms or cirri, within caves or small crevices, beneath rocks, or as attachments via cirri to other invertebrates such as corals (epizoic). Crinoids are important in developmental biology because they are the only echinoderms that have a primitive tripartite coelom. They are significant in paleontology because they evolved during the Cambrian period and were a dominant and diverse component of Paleozoic benthic fauna [6].
Sea cucumbers (Holothuroidea) are mostly found in benthic marine habitats ranging from the littoral zone to the abyss. They are found in all oceans, with particular abundance in coral reefs. Sea cucumbers are primarily detritivores, feeding on both suspended particles and organic matter bound in sediment. The distribution of sea cucumbers is influenced by the type of substrate, as well as other environmental variables such as dominant currents, temperature, water salinity, and depth. Sea cucumbers’ ecological importance is linked to their bioturbation activities, which involve the movement of organic material within sediments as well as the transfer of energy and materials at the water-sediment interface. The commercialization of sea cucumbers’ body walls, also known as beche de mer or trepang, is economically significant. Out of the over a thousand known species, the fishing industry primarily focuses on around thirty. This practice has a long history in Chinese and Japanese culinary and medical traditions. Larger species are especially valuable. Trepang curing is applied to the body wall, resulting in a product with high nutritional value due to its high protein and low fat content. Furthermore, sea cucumbers contain biologically active compounds that are used to treat a variety of medical conditions, including HIV, cancer, and osteoarthritis [7][8].
Echinoids (Echinoidea) are found in a wide variety of geographical and bathymetric environments, from intertidal zones to depths of 5000 m. Regular sea urchins live on a variety of substrates, with the majority of them living on rocky or mobile substrates. Sand dollars and heart urchins, for example, can only be found on soft bottoms and frequently bury themselves. Many morphological differences between regular and irregular echinoids are caused by differences in lifestyle and feeding habits. Some populations of regular sea urchins exposed to wave action have developed digging behaviors to bury themselves slightly, whereas species not exposed to such conditions typically exhibit cryptic behaviors [9]. Sea urchins, as well as sea cucumbers, are valuable species, and the commercialization of sea urchins’ gonads, also known as roe, is economically significant around the world [9][10].
Ophiuroids (Ophiuroidea) have a wide geographic distribution and a benthic lifestyle, having adapted to live in a variety of environments. They have been discovered in submarine hydrothermal vents and on bottoms with cold methane seeps, as well as in intertidal zones and abyssal regions from the tropics to the poles. The majority of species are usually found on soft substrates. They can be carnivorous, scavengers, sediment-consuming, or filter feeders. The majority use multiple feeding methods, though one usually takes precedence over the others. The majority are carnivorous, eating polychaetes, mollusks, and small crustaceans. Because of their diverse feeding strategies, they play an important role in trophic chains. They are not commercially important, unlike some other echinoderm groups. Nonetheless, due to their abundance, they can be used as environmental indicators [11].
Sea stars (Asteroidea) have a wide geographical range and a benthic habitat, inhabiting a variety of marine substrates where they can be abundant and visible. The vast majority are scavengers or opportunistic predators. Many species have generalist feeding habits and play an important role in the structure and functioning of marine communities as apex predators. They play an ecologically important role across latitudes by occupying different levels of the trophic chain, particularly as apex predators in rocky and coral reef ecosystems. This group’s ecological success can be attributed to a variety of morphological and life history characteristics, including indeterminate growth, extraoral and intraoral digestion (providing access to a diverse diet), rapid prey detection and response, and the ability to anchor themselves to substrates using ambulacral tube feet [11][12].
2. Historical Use of Echinoderms
Because sea urchins have been consumed by humans throughout history, the human understanding of this Phylum dates back to prehistoric times [13]. They are depicted in “frescos cretenses”, which date back 4000 years. For a long time, Eastern cultures have consumed and used sea cucumbers for medicinal purposes [14]. The earliest known of these animals dates from the period of Aristotle, who described the first known echinoderm in 350 B.C.E., over 2000 years ago. He described the feeding apparatus of sea urchins in his work “Historia Animalium”, which is now known as Aristotle’s Lantern. It is worth noting that Aristotle classified echinoderms as ostracoderms. The scholars of the time rekindled their interest in nature and began studying these creatures again. Klein coined the term “Echinodermata” in his 1734 work “Naturalis dispositio echinodermatum”. However, he only used it to refer to sea urchins, not all the classes that are now known. Linnaeus classified the genera Asterias, Echinus, and Holothuria as Mollusca in the 10th edition of Systema Naturae (1758). The term “Echinodermata” resurfaced in 1792, when it was recognized that these animals were a distinct group of invertebrates, though sea cucumbers were not included. Later, Lamarck (1809) [15] grouped Echinodermata with the true Coelenterata in the Radiata group of invertebrates. It took nearly four decades for De Tornos (1839) [16] and Salacroux (1840) [17] to coin the term “Echinodermata”. Due to its more advanced structural characteristics, it was argued in 1854 that Echinodermata did not belong with Coelelenterata. Echinodermata have since been recognized as a distinct clade of invertebrates.
Since the nineteenth century, the description of Echinodermata species has been a dominant focus in the literature. Initially, the focus was on species found along Europe’s coasts, as evidenced by Frey and Leuckart’s work in 1847 [18]. As a result of numerous oceanographic expeditions, this trend has expanded to include species from all over the world. While it is impractical to list every expedition and paper that resulted from it, some of the pioneering ones are worth mentioning. HMS Challenger and the Albatross laid the groundwork for hundreds of subsequent expeditions around the world. These expeditions have recently expanded to include deep-sea species as well as those found in intertidal and shallow waters [19][20][21][22][23]. Given that echinoderms have been collected worldwide for over 300 years to describe species, determine distributions, and populate museum collections, it is clear that hundreds of thousands of echinoderms were collected and preserved without much ethical thought.
3. Echinoderms Nervous System
In echinoderms, the nervous system is organized in accordance with the general pentameric pattern of the body plan. Each radius has its own radial nerve cord, which runs the length of the proximo-distal axis and terminates at the distal tip of the arm (in stellate echinoderms) or near the aboral pole (in globose forms). A circumoral nerve ring joins all five individual radial nerve cords at the body’s oral pole to form a single anatomical entity. The radial nerve cords and the nerve ring comprise the echinoderm’s central nervous system (CNS) (Figure 2). This CNS is an anatomically and histologically distinct agglomeration of neurons and glial cells associated with an extensive neuropil (densely interwoven neuronal processes) found nowhere else in the body and is in charge of the initiation and coordination of various body-wide responses [24].
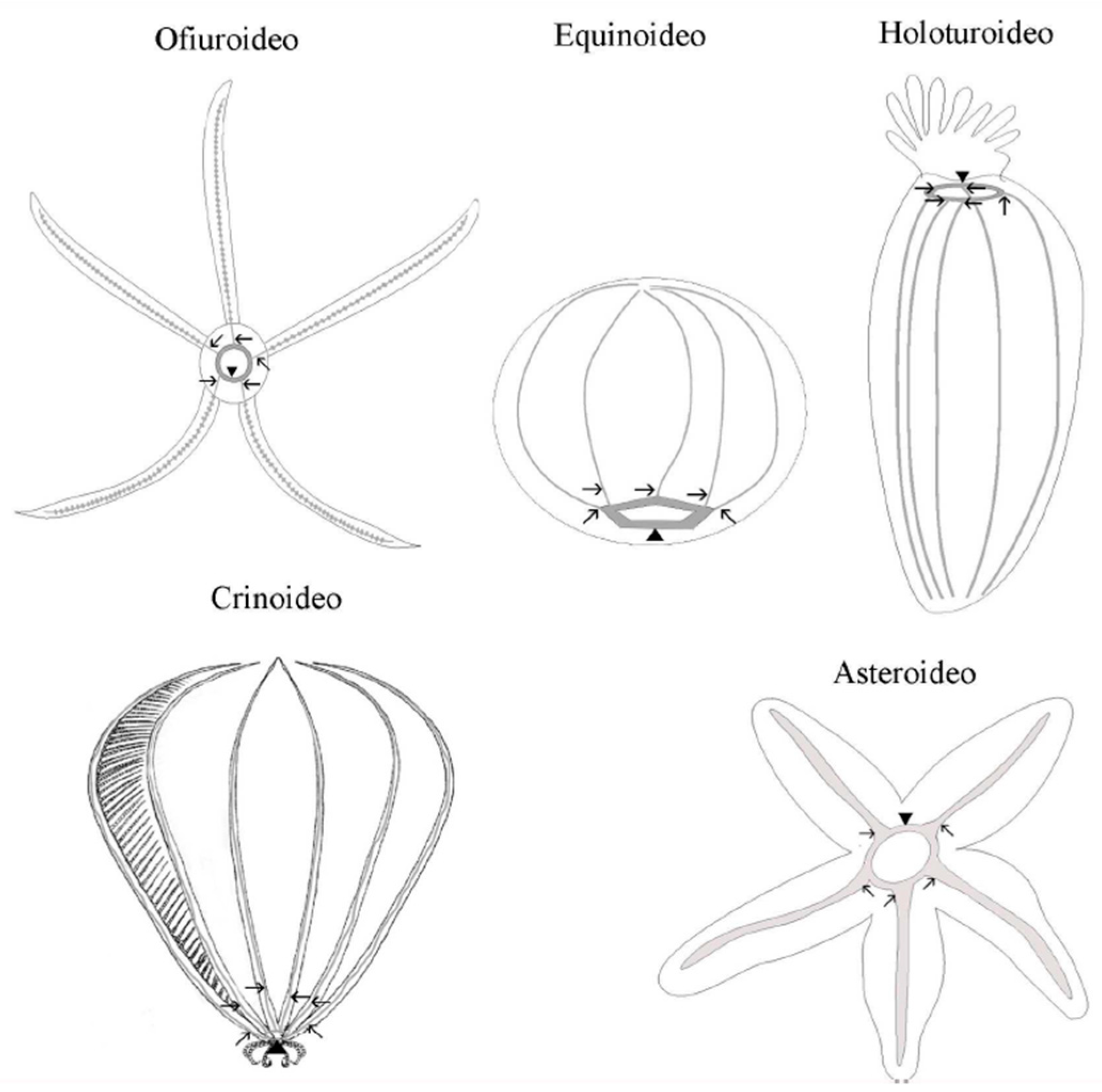
Figure 2. Schematic representation of the central nervous system of the phylum Echinodermata. The arrows indicate the radial nerves cords and the point of the arrows indicates the circumoral ring.
Adult echinoderm neuroanatomy is distinguished by the presence of distinct superimposed domains or layers of nervous tissue located at different levels relative to the oral–aboral axis. These domains are known as the ectoneural and hyponeural systems [25][26][27]. The ectoneural system is located around the mouth, either within or directly beneath the oral epidermis. It is always present both in the nerve ring and radial nerve cords of all echinoderms, shows the most consistent organization across the phylum, and is the predominant part of the nervous system in all classes except crinoids. The hyponeural system may or may not be a part of the nerve ring or radial nerve cords. Its organization differs between classes and is generally related to the degree of development of large muscles. When hyponeural tissue is present, it acts as a second (usually thinner) layer of nervous tissue that directly overlies the aboral surface of the respective ectoneural cords [27][28].
This simplified classification of the echinoderm nervous system, however, does not fully account for recent reports from the last decade, revealing unexpected nervous system elements that do not neatly align with the three aforementioned divisions [24][29]. The nervous system (NS) of echinoderms is one of the most fascinating aspects. Given their close evolutionary relationship with chordates, as well as their radial symmetry and lack of prominent ganglia or centralized nervous structures associated with cephalization found in most other animals, the echinoderm nervous system has been regarded as pivotal to understanding the evolution of the chordate nervous system [4][24]. In fact, it has been suggested that the centralized nervous system is a plesiomorphic (ancestral) condition in echinoderms and may also be a plesiomorphic trait at the level of the Deuterostomia [24].
In the nervous system, including the ectoneural and hyponeural subsystems, tissue is organized as a neuroepithelium made up of two major cell types: radial glial cells and neurons. The cell types have a similar relative abundance in the radial nerve cord’s ectoneural and hyponeural bands, with radial glial cells accounting for 60–70% of the total cell population. On the other hand, neuronal cells are more abundant in the circumoral nerve ring, accounting for only 45% of the cell composition formed by glia [30]. Echinoderm glial cells share significant morphological similarities with the radial glia of chordates, including the orthogonal orientation of the cell’s main axis to the plane of the neuroepithelium, the presence of long thick bundles of intermediate filaments, and the presence of short protrusions branching off at a right angle from the main processes and penetrating into the surrounding neural parenchyma. The cell bodies of the majority of radial glial cells in echinoderms are located at the apical surface of the neuroepithelium. Some of them, however, are bipolar, with the apical and basal processes extending from opposite poles of cell bodies located at different depths within the neural parenchyma. Radial glial cells are the most common type of glial cell in echinoderms (though they are unlikely to be perfectly homogeneous), and they perform a variety of functions. In addition to the radial glia of the CNS, other glial cell types associated with the peripheral nervous system may exist [24]. Radial glia have more morphological similarities than do chordates because they are the main proliferative population in nervous tissue and thus capable of giving rise to new neurons, both in non-injured and regenerating CNSs. There are, however, differences between radial glia in echinoderms and chordates. Another significant difference is that radial glia are the only major glial cell type in the adult CNS of echinoderms, whereas in higher vertebrates, radial glia predominates in embryogenesis but then mostly disappear from the mature nervous system by giving rise to a plethora of more specialized cell types [31][32]. Radial glia are common in the adult CNS of lower vertebrates, but they frequently co-exist with other abundant specialized glia, such as those of oligodendrocytes [33][34].
The neural parenchyma of the CNS is made up of neurons with somata and neurites. The most common neuronal morphology ranges from unipolar and bipolar to multipolar. In echinoderms, neurons can be classified quite easily by size as normal neurons, which are dominant in most classes, and the giant neurons of ophiuroids. The first class has small somata (about 5 m in diameter) that produce very thin give processes (0.1–1 m in cross-section) with numerous local swellings (varicosities) along their length. Because they are typically found near or embedded within calcareous structures, they are less appealing to neurobiologists, particularly electrophysiologists who study their electrical properties. As a result, while other animal groups became popular neurobiology research subjects, echinoderms were largely overlooked for a long time. Even today, there are surprisingly few electrophysiological studies on echinoderms [35][36].
Communication between neurons in traditional chemical synapses was previously thought to be absent in echinoderms [25][37]. However, this long-held belief may be due to a lack of adequate tools for dealing with the difficulties imposed by the endoskeleton. Since the optimization of sample preparation protocols, there has been evidence demonstrating the presence of typical chemical synapses that occur on a regular basis in the CNS of Echinodermata [30][38]. These findings are consistent with those found in the sea urchin genome, where the genes required for synapse formation were discovered [39]. There are also different types of synapses: unsheathing synapses (with the pre-synaptic terminal wrapped around the post-synaptic process), passant synapses between parallel nerve fibers, and complex synapses with a pre-synaptic terminal forming two or more synapses in different post-synaptic processes, or, conversely, a single post-synaptic neuron receiving synaptic input from multiple pre-synaptic axons [38]. Echinoderm CNSs have regional differences in cellular composition as well as a complex internal spatial segregation of different cell types. The radial nerve cord is made up of repetitive units [25][40][41].
The CNS of echinoderms generates complex, coordinated, and directional behavioral responses to various sensory stimuli. Although the molecular and cellular mechanisms underlying these behaviors remain unknown, it is known that an echinoderm’s CNS contains a large number of neurotransmitters from all major groups, including acetylcholine, aminoacids, monoamines, neuropeptides, and gases. Acetylcholine appears to mediate muscle contraction due to its function as a major excitatory neurotransmitter [28][42][43]. Post-synaptic nicotinic and muscarinic receptors have also been identified, and acetylcholinesterase (the enzyme required to hydrolyze acetylcholine at synapses) activity has been detected in both ectoneural and hyponeural systems [25][44]. GABAergic neurons proliferate throughout the CNS, including the radial nerve cord, nerve ring, and podial nerves, as well as the nerves and visceral plexi [24][45]. GABA is involved in both echinoderm muscle contraction and relaxation, depending on the post-synaptic receptor (GABA A or GABA B) present in neuromuscular junctions [42]. L-glutamate is and excitatory neurotransmitter in the ectoneural subsystem of echinoderms [46][47]. L-glutamate is also a neurotransmitter capable of eliciting the arm autotomy response, whereas acetylcholine acts as an antagonist of L-glutamate [46]. The research on serotonin as a neurotransmitter in the adult CNS is limited. There have been reports of its presence in muscles and basiepithelial plexi [48][49][50]. Furthermore, neuropharmacological studies have revealed that serotonin regulates muscular contraction by inhibiting the excitatory effect of acetylcholine [49] and may also be involved in the regulation of post-traumatic regeneration [50]. Catecholamines such as dopamine and noradrenaline have been found in the ectoneural only and appear to be involved in the movement of tube feet [51][52] and to be fundamental for the righting response of the sea urchin [53]. Histamine data of the sea urchin are extremely limited; they have only been studied in one species of cucumber, and the latter appears to be involved in sensory systems, as it was found in tentacles and body wall papillae, and to project its axons directly to the nerve ring and the radial nerve cord [54].
The enzyme nitric oxide synthase, which produces NO, was discovered in both the ectoneural and hyponeural parts of the radial nerve cord of adult sea stars, as well as in some radial glial cells [55][56]. Apparently, NO is involved in the relaxation of viscera and tube feet [57][58].
In addition to these phylogenically widespread neurotransmitters, echinocherms contain specific neuropeptides from the SALMFamide family [28][40][58][59][60][61]. These neuropeptides relax the visceral musculature as well as the muscles of the body wall [58][62].
All of the information above suggests that the echinoderm CNS is more complex than previously thought and that, despite the lack of a centralized brain, it is possible for it to elicit complex individual and social behavior. Recent advances in knowledge of this group have provided new insights suggesting that echinoderms are sentient animals capable of suffering pain.
4. Pain and Echinoderms
Even though the debate over pain perception in invertebrates is still ongoing, it is critical to recognize that the absence of evidence of painful sensations should not be interpreted as conclusive proof of pain absence in this group.
Various agents have been used to anesthetize echinoderms [63]. Iso-osmotic solutions such as MgCl2, MgSO4, or Ca2+-free seawater are common echinoderm anesthetics. The mechanism of action of these agents is to destabilize membrane potential, preventing pain signals from propagating. Additionally, local anesthetics that block neural stimulation of the muscle have been employed when necessary. The local anesthetics MS222 [61][64][65][66][67] and propylene phenoxetol [64][68][69][70][71][72][73] have both demonstrated effectiveness in studies involving echinoderm connective tissue.
MS-222 (IUPAC name 3-amino benzoic acid, ethyl ester, and methanesulfonate salt, also known as ethyl m-aminobenzoate or tricaine methanesulfonate) is a local anesthetic of the ester type. Its structure is similar to that of other local anesthetics, such as benzocaine, implying that it likely functions similarly by impeding axonal conduction via interference with membrane depolarization [74]. Originally developed as a fish anesthetic [75], it has since been widely used on a variety of invertebrates (National Research Council, 1981).
In ophiuroids, “propylene phenoxetol” is thought to act as a local anesthetic that inhibits axonal conduction [73]. Because this volatile liquid is not water-soluble, maintaining known concentrations of the compound in a medium is difficult. Furthermore, there have been some doubts about the precise identity of this compound. While certain compounds possessing anesthetic effects in echinoderms have been studied, the practice of using them during individual manipulations is not yet standardized because there is neither awareness nor a normative mandate for it.
This entry is adapted from the peer-reviewed paper 10.3390/ani13213377
References
- Brusca, R.C.; Moore, W.; Schuster, M. Invertebrates; Sinauer Associated, Inc.: Sunderland, MA, USA, 2016.
- Telford, M.J.; Lowe, C.J.; Cameron, C.B.; Ortega-Martinez, O.; Aronowicz, J.; Oliveri, P.; Copley, R.R. Phylogenomic Analysis of Echinoderm Class Relationships Supports Asterozoa. Proc. R. Soc. B 2014, 281, 20140479.
- Smith, A.B. Echinoderms (Other Than Echinoids). In Encyclopedia of Geology; Cocks, R., Plimer, I., Eds.; Elsevier: Oxford, UK, 2005; pp. 334–341.
- Arnone, M.I.; Byrne, M.; Martinez, P. Echinodermata. In Evolutionary Developmental Biology of Invertebrates 6; Wanninger, A., Ed.; Springer: Vienna, Austria, 2015.
- Amemiya, C.T.; Miyake, T.; Rast, J.P. Echinoderms. Curr. Biol. 2005, 15, R944–R946.
- Hess, H.; Messing, C.; Ausich, W.I. Treatise on Invertebrate Paleontology; The University of Kansas Paleontological Institute: Lawrence, KS, USA, 2011.
- Chen, J. Overview of Sea Cucumber Farming and Sea Ranching Practices in China. SPC Beche-de-mer Inf. Bull. 2003, 18, 18–23.
- Slater, M.; Chen, J. Sea Cucumber Biology and Ecology. In Echinoderm Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 47–55.
- Lawrence, J.M. (Ed.) Sea Urchins: Biology and Ecology; Academic Press: Cambridge, MA, USA, 2013.
- Sun, J.; Chiang, F.S. Use and Exploitation of Sea Urchins. In Echinoderm Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 25–45.
- López-Pérez, A.; Granja-Fernández, R.; Aparicio-Cid, C.A.; Zepeta-Vilchis, R.C.; Torres-Huerta, A.M.; Benítez-Villalobos, F.; López-López, D.A.; Cruz-Antonio, C.; Valencia-Méndez, O. Corales Pétreos, Equinodermos y Peces Asociados a Comunidades y Arrecifes Coralinos del Parque Nacional Huatulco, Pacífico Sur Mexicano. Rev. Mex. Biodivers. 2014, 85, 1145–1159.
- Luna Salguero, B.; Bonilla, H. Estructura Comunitaria y Trófica de las Estrellas de Mar (Echinodermata: Asteroidea) en Arrecifes Rocosos de Loreto, Golfo de California, México. Hidrobiológica 2010, 20, 127–134.
- Lawrence, J.M. Sea Urchin Roe Cuisine. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, pp. 521–523.
- Brown, N.; Eddy, S. Echinoderm Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2015.
- Lamarck, J.B. Philosophie Zoologique, ou, Exposition des Considérations Relative à l’Histoire Naturelle des Animaux; Librairie F. Savy: Paris, France, 1809.
- De Tornos, L. Compendio de Historia Natural Dividido en los Tres Ramos de Mineralogía, Botánica y Zoología; Imprenta Salvador Albert: Madrid, Spain, 1839.
- Salacroux, A.P.G. Nuevos Elementos de Historia Natural; Imprenta de Verges: Madrid, Spain, 1840.
- Frey, H.; Luckart, R. Zootomie; Voss: Leipzig, Germany, 1847.
- Agassiz, A. Report on the Echinoidea Dredged by H.M.S. Challenger during the Years 1873–1876. Zoology Part IX Challenger Reports; Johnson Reprint Corporation: New York, NY, USA, 1881; Volume 3, pp. 1–321.
- Théel, H. Report on the Holothurioidea Dredged by H.M.S. Challenger during the Years 1873–1876. Zoology Part IX Challenger Reports; Johnson Reprint Corporation: New York, NY, USA, 1882.
- Agassiz, A. Notice of Calamocrinus Diomedeae, a New Stalked Crinoid from the Galapagos, Dredged by the U. S. Fish Commission Steamer ‘Albatross’. Bull. Museum Comp. Zool. 1890, 20, 165–167.
- Clark, H.L. Papers from the Hopkins Stanford Galapagos Expedition. Proc. Wash. Acad. Sci. 1902, 4, 521–531.
- De Morgan, W. The Echinoderms Collected by the “Huxley” from the North Side of the Bay of Biscay in August, 1906. J. Mar. Biol. Assoc. 1913, 9, 530–541.
- Mashanov, V.; Zueva, O.; Rubilar, T.; Epherra, L.; García-Arrarás, J.E. Echinodermata. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 665–688.
- Cobb, J.L.S. Neurobiology of the Echinodermata. In Nervous Systems in Invertebrates; Breidbach, O., Kutsch, W., Eds.; Springer US: Boston, MA, USA, 1987; pp. 483–525.
- Heinzeller, T.; Welsch, U. The Echinoderm Nervous System and Its Phylogenetic Interpretation. In Brain Evolution and Cognition; Roth, G., Wullimann, M., Eds.; Wiley-Spektrum: New York, NY, USA, 2001; pp. 41–75.
- Hyman, L. The Invertebrates. IV. Echinodermata. The Coelomate Bilateria; McGraw-Hill Book Co.: New York, NY, USA, 1955.
- Heinzeller, T.; Welsch, U. Crinoidea. In Microscopic Anatomy of Invertebrates; Harrison, F., Chia, F.-S., Eds.; Wiley-Liss: New York, NY, USA, 1994; Volume 14, pp. 1–148.
- Díaz-Balzac, C.A.; García-Arrarás, J.E. Echinoderm Nervous System. In Oxford Research Encyclopedia of Neuroscience; Oxford University Press: Oxford, UK, 2018.
- Mashanov, V.S.; Zueva, O.R.; Garcia-Arraras, J.E. Organization of Glial Cells in the Adult Sea Cucumber Central Nervous System. Glia 2010, 58, 1581–1593.
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184.
- Malatesta, P.; Appolloni, I.; Calzolari, F. Radial Glia and Neural Stem Cells. Cell Tissue Res. 2008, 331, 165–178.
- Tanaka, E.M.; Ferretti, P. Considering the Evolution of Regeneration in the Central Nervous System. Nat. Rev. Neurosci. 2009, 10, 713–723.
- Zamora, A.J. The Ependymal and Glial Configuration in the Spinal Cord of Urodeles. Anat. Embryol. 1978, 154, 67–82.
- Binyon, J.; Hasler, B. Electrophysiology of the Starfish Radial Nerve Cord. Comp. Biochem. Physiol. 1970, 32, 747–753.
- Millott, N.; Okumura, H. The Electrical Activity of the Radial Nerve in Diadema antillarum Philippi and Certain Other Echinoids. J. Exp. Biol. 1968, 48, 279–287.
- Cobb, J. The Nervous Systems of Echinodermata: Recent Results and New Approaches. In The Nervous Systems of Invertebrates: An Evolutionary and Comparative Approach; Breidbach, O., Kutsch, W., Eds.; Birkhhäuser Verlag: Basel, Switzerland, 1995; pp. 407–424.
- Mashanov, V.; Zueva, O.; Heinzeller, T.; Dolmatov, I. Ultrastructure of the Circumoral Nerve Ring and the Radial Nerve Cords in Holothurians (Echinodermata). Zoomorphology 2006, 125, 27–38.
- Burke, R.D.; Angerer, L.M.; Elphick, M.R.; Humphrey, G.W.; Yaguchi, S.; Kiyama, T.; Liang, S.; Mu, X.; Agca, C.; Klein, W.H.; et al. A Genomic View of the Sea Urchin Nervous System. Dev. Biol. 2006, 300, 434–460.
- De Bremaeker, N.; Deheyn, D.; Thorndyke, M.C.; Baguet, F.; Mallefet, J. Localization of S1- and S2-like Immunoreactivity in the Nervous System of the Brittle Star Amphipholis squamata (Delle Chiaje 1828). Proc. R. Soc. B Biol. Sci. 1997, 264, 667–674.
- Cobb, J.L.; Stubbs, T.R. The Giant Neurone System in Ophiuroids. I. The General Morphology of the Radial Nerve Cords and Circumoral Nerve Ring. Cell Tissue Res. 1981, 219, 197–207.
- Devlin, C.L. The Pharmacology of Gamma-Aminobutyric Acid and Acetylcholine Receptors at the Echinoderm Neuromuscular Junction. J. Exp. Biol. 2001, 204, 887–896.
- Florey, E.; Cahill, M.A. Cholinergic Motor Control of Sea Urchin Tube Feet: Evidence for Chemical Transmission without Synapses. J. Exp. Biol. 1980, 88, 281–292.
- Ryberg, E. The Localization of Cholinesterases and Non-Specific Esterases in the Echinopluteus. Zool. Scr. 1974, 2, 163–170.
- Newman, S.J.; Thorndyke, M.C. Localisation of Gamma Aminobutyric Acid (GABA)-Like Immunoreactivity in the Echinoderm Asterias rubens. Cell Tissue Res. 1994, 278, 177–185.
- Wilkie, I.C.; Barbaglio, A.; Maclaren, W.M.; Carnevali, M.D.C. Physiological and Immunocytochemical Evidence That Glutamatergic Neurotransmission Is Involved in the Activation of Arm Autotomy in the Featherstar Antedon mediterranea (Echinodermata: Crinoidea). J. Exp. Biol. 2010, 213, 2104–2115.
- Wilkie, I.C.; Barbaglio, A.; Carnevali, M.D.C. The Elusive Role of L-Glutamate as an Echinoderm Neurotransmitter: Evidence for Its Involvement in the Control of Crinoid Arm Muscles. Zoology 2013, 116, 1–8.
- Candia Carnevali, M.; Bonasoro, F.; Thorndyke, M.C.; Patruno, M. Role of the Nervous System in Echinoderm Regeneration. In Echinoderms 2000; Barker, M., Ed.; Balkema: Rotterdam, The Netherlands, 2001; pp. 5–20.
- Inoue, M.; Tamori, M.; Motokawa, T. Innervation of Holothurian Body Wall Muscle: Inhibitory Effects and Localization of 5-HT. Zool. Sci. 2002, 19, 1217–1222.
- Sugni, M.; Ferreri, F.; Bonasoro, F.; Candia Carnevali, M.; Wilkie, I.C. New Evidence for Serotonergic Control of Regenerative Processes in Crinoids. In Echinoderms: München; Heinzeller, T., Nebelsick, J., Eds.; Taylor & Francis Group: London, UK, 2004; pp. 141–146.
- Cottrell, G.A.; Pentreath, V.W. Localization of Catecholamines in the Nervous System of a Starfish, Asterias rubens, and of a Brittlestar, Ophiothrix fragilis. Comp. Gen. Pharmacol. 1970, 1, 73–81.
- Díaz-Balzac, C.A.; Mejías, W.; Jiménez, L.B.; García-Arrarás, J.E. The Catecholaminergic Nerve Plexus of Holothuroidea. Zoomorphology 2010, 129, 99–109.
- Howell, E.; Lancaster, A.; Besh, J.; Richardson, B.; Gomez, E.; Harnew-Spradley, S.; Shelley, C. The Dopamine Receptor Antagonist Haloperidol Disrupts Behavioral Responses of Sea Urchins and Sea Stars. J. Exp. Biol. 2023, 226, 17.
- Hoekstra, L.A.; Moroz, L.L.; Heyland, A. Novel Insights into the Echinoderm Nervous System from Histaminergic and FMRFaminergic-Like Cells in the Sea Cucumber Leptosynapta clarki. PLoS ONE 2012, 7, e44220.
- Kotsiuba, E.P.; Kotsiuba, A.E. NADPH-Diaphorase Localization in the Radial Nerve Cords of the Starfish Patiria pectinifera. Tsitologiia 2004, 46, 346–351.
- Martinez, A.; Riveros-Moreno, V.; Polak, J.M.; Moncada, S.; Sesma, P. Nitric Oxide (NO) Synthase Immunoreactivity in the Starfish Marthasterias glacialis. Cell Tissue Res. 1994, 275, 599–603.
- Elphick, M.R.; Melarange, R. Nitric Oxide Function in an Echinoderm. Biol. Bull. 1998, 194, 260–266.
- Elphick, M.R.; Melarange, R. Neural Control of Muscle Relaxation in Echinoderms. J. Exp. Biol. 2001, 204, 875–885.
- Beer, A.J.; Moss, C.; Thorndyke, M. Development of Serotonin-Like and SALMFamide-Like Immunoreactivity in the Nervous System of the Sea Urchin Psammechinus miliaris. Biol. Bull. 2001, 200, 268–280.
- Elphick, M.R.; Achhala, S.; Martynyuk, N. The Evolution and Diversity of SALMFamide Neuropeptides. PLoS ONE 2013, 8, e59076.
- O’Neill, P.L. Structure and Mechanics of Starfish Body Wall. J. Exp. Biol. 1989, 147, 53–89.
- Elphick, M.R.; Kemenes, G.; Staras, K.; O’Shea, M. Behavioral Role for Nitric Oxide in Chemosensory Activation of Feeding in a Mollusc. J. Neurosci. 1995, 15, 7653–7664.
- Kaplan, H.M. Anesthesia in Invertebrates. Fed. Proc. 1969, 28, 1557–1569.
- Wilkie, I.C. Nervously Mediated Change in the Mechanical Properties of a Brittlestar Ligament. Mar. Behav. Physiol. 1978, 5, 289–306.
- O’Neill, P.L. Torsion in the Asteroid Ray. J. Morph 1990, 203, 141–149.
- O’Neill, P.L. The Effect of Anaesthesia on Spontaneous Contraction of the Body Wall Musculature in the Asteroid Coscinasterias calamaria. Mar. Behav. Physiol. 1994, 24, 137–150.
- Motokawa, T.; Wainwright, S.A. Stiffness of Starfish Arm and Involvement of Catch Connective Tissue in the Stiffness Change. Comp. Biochem. Physiol. 1991, 100A, 393–397.
- Wilkie, I.C. Nervously Mediated Change in the Mechanical Properties of the Cirral Ligaments of a Crinoid. Mar. Behav. Physiol. 1983, 9, 229–248.
- Wilkie, I.C. Variable Tensility of the Oral Arm Plate Ligaments of the Brittlestar Ophiura ophiura (Echinodermata: Ophiuroidea). J. Zool. 1992, 228, 5–26.
- Byrne, M. The Mechanical Properties of the Autotomy Tissues of the Holothurian Eupentacta quinquesemita and the Effects of Certain Physico-Chemical Agents. J. Exp. Biol. 1985, 117, 69–86.
- Byrne, M. Induction of Evisceration in the Holothurian Eupentacta quinquesemita and the Evidence for the Existence of an Endogenous Evisceration Factor. J. Exp. Biol. 1986, 120, 25–40.
- Wilkie, I.C.; Emson, R.H.; Mladenov, P.V. Morphological and Mechanical Aspects of Fission in Ophiocomella ophiactoides (Echinodermata, Ophiuroidea). Zoomorphology 1984, 104, 310–332.
- Wilkie, I.C.; Griffiths, G.V.R.; Glennie, S.F. Morphological and Physiological Aspects of the Autotomy Plane in the Aboral Integument of Asterias rubens L. (Echinodermata). In Echinoderm Research; De Ridder, C., Dubois, P., Lahaye, M.-C., Jangoux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1990; pp. 301–313.
- MacDonald, A.G.; Wann, K.T. Physiological Aspects of Anaesthetics and Inert Gases; Academic Press: London, UK, 1978.
- Jolly, D.W.; Mawdsley-Thomas, L.E.; Bucke, D. Anaesthesia of Fish. Vet. Rec. 1972, 91, 424–426.
This entry is offline, you can click here to edit this entry!
