Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
The skin demonstrates complicated electrical characteristics that involve physiological and physical components in terms of its physical and chemical condition. The electrical features of the skin are of significant importance in bioengineering applications in wound healing given that physical attributes such as impedance and conductance reflect the composition of the skin. Electrical aspects of skin are broadening the spectrum of applications of the technologies for assessing and evaluating skin barrier integrity and the wound-healing process.
- wound healing
- Skin
- Tissue regeneration
- Bioelectric factors
1. Bioelectrical Properties in Normal Skin
The electrical characteristics of normal skin are the primary focus of bioimpedance research. Ions are the major charge carrier in biological materials [63]. The electrical conductivity of skin tissue is determined by multiple factors, including electric field frequency, tissue structure, charge carrier mobility, and is regulated by electrons and ions [64,65].
1.1. Epidermal Ion Distribution
The skin contains an inherent bioelectric system that generates endogenous electrochemical signals [66]. Electrical potential energy is produced by asymmetrical ionic fluxes across tissue layers of the skin via the sodium–potassium pump in the epidermis (Figure 1a) [67]. Due to the tightly connected keratinocytes comprising the epidermis, there is a higher level of ion transport within cells and less diffusion in the surrounding tissues [68]. Several studies have indicated the presence of divalent ions including calcium and magnesium, which exhibit noticeable peaks at the boundary between the epidermis and the stratum corneum [69,70]. Sodium and chloride are dispersed uniformly throughout the epidermis; however, potassium levels decline significantly from the stratum granulosum to the bottom layers [71]. Calcium has been identified as a significant signaling ion, as its distribution aligns with the distinct requirements of each skin layer at individual differentiation stages. Low calcium concentrations in deeper skin layers stimulate proliferative activity, while high concentrations near top layers differentiate and secrete lamellar bodies, thereby creating a functional skin barrier [72,73].
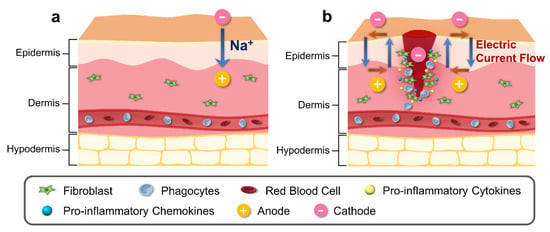
Figure 1. Schematic illustration of physiological and bioelectrical factors of normal and wound skin. (a) Electric potential of normal skin is generated by asymmetric ion fluxes across the tissue layers through the Na⁺/K⁺ pump in the epidermis. (b) Injury to skin generates cathode in the center of the wound, disrupting the regular current flow. Difference in electric potential between the edges of the wound and the center of the wound creates a current flow that stimulates cell migration to the wound site for increased proliferation and phagocytosis, with release of pro-inflammatory cytokines and chemokines.
1.2. Transepithelial Potential (TEP)
The gradient of ions across the skin tissue creates a potential difference called the transepithelial potential (TEP), also known as the ‘skin battery’, along the depth of the epidermis [74]. The voltage difference between the stratum corneum and the dermis in humans has been reported to average −23 mV, with variations depending on anatomical location [75]. Early research centered on the connection across different electrical properties of the skin along with the psychological reaction being relevant to sweat gland activity [76,77]. Subsequently, a potential difference produced independently across the epidermis was identified, which stimulated the attention of researchers in the involvement of ion transport in the electrophysiological system of epidermal keratinocytes. TEP values ranging from 10 to 60 mV have been measured on intact epidermis; however, due to the relatively low number of tissues under the dermis, this results in weakly retained ionic gradients and a small potential difference [78,79].
1.3. Bioimpedance and Barrier Properties of Skin
The stratum corneum influences the overall skin impedance mainly through its hydrophobic properties, which act as a permeability barrier, as the applied current is conducted through the skin tissue by the water-soluble charged molecular components present [80]. Multi-layered skin models are often described as a mixture of comparable circuits composed of living tissues associated with ionic molecules and their motilities [81]. The impedance properties are essential for effective biomimetic design in terms of transdermal monitoring of current flow.
Like many other biological tissues, the skin displays dielectric characteristics [82]. When dealing with low frequencies, the skin exhibits an increased resistance, while at higher frequencies, it is reduced due to its capacitive properties [83,84]. Additionally, applying high voltage pulses on skin tissue can cause electroporation, which increases cell permeability, as well as electroosmotic flow and thermal perturbation, which causes a temporary or permanent shift in molecular dynamics, boosting skin conductivity [85,86].
2. Bioelectrical Factors during Wound Healing
2.1. Alteration in Epidermal Ion Distribution and Electric Potential
The ionic composition of the skin has been observed to be influenced by the infiltration of exogenous substances [87]. In normal skin, the epidermal electrical potential is generated by asymmetric ion fluxes that continuously pump Na⁺ ions within the tissue layers via the Na⁺/K⁺ pump (Figure 1b). However, during the process of wound healing or skin injury, the current generated from the skin undergoes alteration (Figure 1b). During the process of wound healing or skin injury, the current generated from the skin undergoes alteration (Figure 1b). The electrical potential created by the wounded skin, which is directed towards the area of the wound and has a cathode in the center and an anode at the edge, attracts cells to the injured area [88]. This potential is created by the wounded skin’s current, and the longitudinal electric field created by this current will last until the resistance rises throughout the healing process [89]. The variables that influence TEP and ion distribution following a temporary wound or skin barrier disruption subsequently return to normal levels through a combination of passive diffusion and active transport in the tissue during wound healing [90].
When calcium levels in the stratum granulosum are diminished, barrier restoration mechanisms are activated, which involves the secretion of lamellar bodies into the intercellular gaps of the stratum corneum to replenish them with lipids [72]. Furthermore, calcium also plays a crucial role in the formation of desmosomes and migration of cells in the wound-healing process [91]. In the early phases of wound healing, desmosomal adhesion is regulated in a calcium-dependent manner permitting the cell movement, and over time, providing mechanical support for the regenerating epithelium [92].
2.2. Electric Field Profiles in Wound Sites
One of early studies utilized a galvanometer to measure roughly 1 µA emanating from a cut in a finger, which gave rise to current research on an electric field resulting from a wound [93]. In another study of human fingertip amputation currents, the vibrating probe technique was used to measure the leakage current across epithelial tissue in low-resistance regions, with a maximum value of up to 30 A/cm2 [94]. In a different study, a vibrating probe was used to measure the lateral electric field of a lancet wound [95]. The reported results showed that the electric field in 18- to 25-year-olds was on average 107 to 148 mV/mm, which was 48% larger than that in 65- to 80-year-olds, demonstrating an age-related influence on the electric field within the region between the epidermis and stratum corneum. [95]. In a mouse wound model, the mean value of the lateral electric field immediately after wound formation was 122 ± 9 mV/mm, and after the wound site was filled with organized and dense epidermal layers, the mean value decreased to 59 ± 5 mV/mm, suggesting that the variance in electrical surface potential is greater in wounds and decreases gradually as the skin returns to its normal state [95].
2.3. Effects of Electric Field Alteration on Cell Components
Keratinocytes move towards the cathode at a speed of approximately 1 µm/min when subjected to electric fields of 100 mV/mm [78,96]. In vitro, a direct current of 200 mV/mm, which is comparable to the electrophysiological field strength of skin wounds, attracts keratinocytes to the cathode pole and causes autophagy [97]. Keratinocytes are also propelled toward the cathode by a pulsed electric stimulation of at least 150 mV/mm independently of the frequency of the pulses [98]. Another study employing an electric field of 50 mV/mm, reported the migration of keratinocytes in the direction of the anode [99]. This observation of keratinocyte migration to the anode appears to contradict previous findings and may be due to differences in keratinocyte integrin expression, an important factor in wound healing as integrins are involved in the regulation of keratinocyte functions [100]. The axial positions of fibroblasts are parallel to the electric field [101,102]. When fibroblasts are exposed to an electric field of at least 100 mV/mm, collagen synthesis accelerates by approximately 100% and DNA synthesis enhances by 20%, resulting in efficient wound healing [103]. Furthermore, a physiological electrodynamic field attracts macrophages to the anode pole, increasing their capacity for phagocytosis, whereas their progenitor, the monocyte, is drawn to the cathode pole [104]. Thus, electric fields are thought to play a significant role in the overall wound-healing process through their effects on cell migration and the immune system.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8070517
This entry is offline, you can click here to edit this entry!
