1. Introduction
Metabolic pathways are tightly regulated [
71], and many pathological conditions, including obesity, diabetes, hypertension, heart disease, and cancer, are associated with abnormal metabolic states [
72,
73]. The cluster of interrelated adverse metabolic markers of hyperglycemia, dyslipidemia, and hypertension, alongside central or abdominal obesity, is termed the “metabolic syndrome”, for which numerous strategies have been tested, including pharmacological treatments, physical exercise, and dietary regimens [
74].
Nutraceuticals, assumed as a food supplement, besides serving as dietary nutrients, can act as cis- or trans-regulators of the human metabolome. Many of these functions occur through both the direct regulation of receptor-mediated cellular signaling, mainly in enterocytes and infiltrating immune cells, and the indirect boosting of mucosal microbiota, thus potentially ameliorating intestinal barrier integrity and homeostasis [
75]. Other biological activities are carried out by peptide sequences encrypted inside a protein nutraceutical, which exert their actions when released, mainly enzymatically, during food processing, digestion, or microbial fermentation [
76].
Whey proteins, as key constituents of milk-based products, have been assessed for their contribution to the potential beneficial effects of dairy consumption, as evidenced from studies which have demonstrated the efficacy of whey proteins in counteracting obesity-related pathologies. The ingestion of milk-derived proteins can affect plasma lipid levels; in particular, whey protein tends to lower plasma lipid levels [
77] and to enhance weight and body-fat loss during energy restriction and limited hepatic fat accumulation [
78]. Similarly, Zapata and colleagues found that whey proteins and, even more so, the isolated components lactalbumin (La) and Lf, were able to induce sustained weight and fat loss and to ameliorate the energy expenditure in diet-induced obese rats, together with decreasing the levels of plasma leptin and insulin and improving glucose clearance, as well as decreasing hepatic lipidosis [
79].
Among the whey proteins, Lf plays a central role in being naturally able to interact with host cell receptors and with microbial pathogen-associated molecular patterns (PAMPs), thus exerting a global multitargeted action. At variance with other whey proteins, Lf is also endogenously expressed by specialized host cells, hence representing a self-defense system which can be turned on/off depending on the metabolic circumstances. However, the tissue or plasma Lf concentrations required to exert a specific biological activity are hardly reached in vivo, therefore requiring additional administration from exogenous sources.
The first evidence for the association between endogenous circulating Lf and metabolic disorders was provided by two studies by Fernandez-Real and colleagues, who analyzed the concentration of circulating Lf in patients suffering from insulin resistance and altered glucose tolerance (AGT) [
80,
81]. In humans, both circulating Lf and Lf gene polymorphisms were shown to be associated with dyslipidemia and vascular reactivity in patients with AGT [
80]. Moreover, the mean circulating Lf was significantly higher in healthy subjects than in AGT subjects, and it positively correlated with insulin sensitivity and negatively with age, fasting glucose, and glycated hemoglobin levels [
81]. Interestingly, opposite results were obtained by Mayeur and colleagues, who reported a positive correlation between serum Lf and insulin resistance in a cohort of lean to moderately obese women [
82]. Similarly, an increased baseline concentration of serum Lf, reflecting neutrophil priming caused by hyperglycemia, was described as a predictor of the long-term risk of fatal ischemic heart disease in newly diagnosed diabetes subjects [
83]. Few studies have investigated the potential correlation between endogenous Lf changes in humans presenting with lipid metabolic disorders. A study on subjects with morbid obesity demonstrated a significant inverse association of circulating Lf with postprandial lipemia and oxidative stress after acute fat intake. Subjects showing the highest increase in serum Lf presented the lowest changes in free fatty acids (FFAs), high-density lipoprotein cholesterol (HDL-C), as well as in C-reactive protein and antioxidant enzymes such as catalase and glutathione reductase, suggesting an ameliorated response to fat load in those subjects with increased endogenous Lf [
84]. On the other hand, no significant difference in serum Lf concentrations was found in metabolically healthy and unhealthy obese women, with only minor correlations with some anthropometric and metabolic parameters [
85].
Globally, these contradictory results can be due to differences in sex, age, and related pathologies, or linked to the applied analysis and statistics. Moreover, most of these studies did not properly consider the contribution of exogenous sources from an individual diet regimen to Lf availability and levels in the gastrointestinal tract and blood circulation, thus posing a serious limitation to comparative analysis. Interestingly, Lf and Lf receptor gene variants have been associated with the prevalence of disorders in glucose and lipid metabolism. Two Lf gene polymorphisms (LTF rs1126477 and rs1126478) were reported to be associated with HDL-C and triglyceride (TG) levels in subjects with AGT [
80], whereas significant differences in low-density lipoprotein cholesterol (LDL-C) levels between LTF rs1126477 gene variants were found in metabolically healthy obese (MHO) subjects [
86]. A gene polymorphism in LRP1 (rs4759277) was also associated with fasting insulin levels and homeostasis model assessment of insulin resistance in patients with metabolic syndrome [
87], whereas significant differences in waist circumference and HDL-C levels among rs4759277 gene variants were reported in MHO patients [
86]. Taken together, these data do not support an exhaustive conclusion, and it is difficult to form a clear link between LTF variants and metabolic disorders. Hence, more approaches are still required to better clarify if an actual correlation between fluctuations in endogenous Lf levels and metabolic disorders exists and what the physiological significance of this could be.
More robust evidence for the effects of the exogenous administration of Lf on energy metabolism was shown in several in vitro animal models and a few clinical studies. Among the multiple intricate pathways constituting human metabolism, glucose and lipid metabolisms were the most investigated.
2. Lactoferrin and Glucose Metabolism
Human tissues depend on glucose as the major energy source, but excessive consumption can trigger acute and chronic metabolic disorders. Production and release of pancreatic hormones, mainly insulin and glucagon, ensures balanced blood levels of glucose.
In this scenario, Lf is intriguing as it can regulate glucose metabolism through both cis- and trans-acting mechanisms, which encompass the direct binding of circulating sugars or the upstream modulation of metabolic pathways. In early 2010, Mir and colleagues demonstrated the presence of a sugar-binding site in bLf, with a K
d for glucose in the range of 10
−4–10
−5 M [
88]. Crystallographic studies showed that glucose and other sugars bind to bLf within an elongated shallow cleft on the surface of the C-lobe through several hydrogen bonds and van der Waals interactions [
88]. The site has mixed hydrophilic and hydrophobic features, with polar residues (Thr, His, and Glu) in proximity to the two ends and non-polar residues (Pro and Gly) interspersed within the cleft. Of note, the researchers showed that purified bLf C-lobe significantly decreased the level of free serum glucose in human blood in vitro. A deeper analysis of the effect of glucose binding to bLf on conformational changes and thermodynamic stability of the glycoprotein showed that glucose induces conformational changes in bLf like those observed in the typical heat-induced two-step denaturation process, with an initial increase in α-helix and β-sheet contents of 6% and 14%, respectively [
89]. Interestingly, glucose impaired the proliferative effect of bLf toward MC 3T3-E1 cells in a dose-dependent manner, thus suggesting that free sugar can influence Lf biological activities [
89]. To our knowledge, no sugar-binding properties have been shown for other members of the transferrin (Tf) family, suggesting that the mechanism selectively evolved in Lf, whose endogenous expression is firmly regulated by stress conditions. Overall, Lf’s ability to directly bind mono- and disaccharides could be exploited to reduce the presence, and thus the absorption, of free sugars in the gut during meals; however, more efforts are needed to clarify this hypothesis.
More reports have investigated the efficacy of exogenously administered Lf in alleviating disorders of glucose homeostasis. First, hLf was found to significantly boost the insulin-induced response, in terms of
473SerAkt phosphorylation, in human hepatocarcinoma HepG2 and in non-differentiated and pre-differentiated 3T3-L1 fibroblast mouse cell lines under non-inflammatory and inflammatory conditions [
90]. At a dose of 1 μM and higher, hLf showed insulinotropic activity, increasing Akt phosphorylation on serine 473 in conditions where insulin action was blunted, such as in pre-differentiated 3T3-L1 cells and in cells treated with pro-inflammatory media [
90]. The potential of Lf in counteracting glucose disorders has been shown in two different studies by the group of Takeuchi, who investigated the effect of bLf administration in rats under physiological or hyperglycemia-mimicking conditions. Intraperitoneal administration of 100 mg/kg bLf to rats before an oral glucose-tolerance test (OGTT), following 60 min of restraint stress (RS) load, significantly lowered plasma glucose transition when compared with a vehicle-treated group. In parallel, bLf decreased plasma corticosterone, an RS-induced glucocorticoid known to promote glucose intolerance, while not affecting plasma levels of glucagon and insulin [
91]. These findings suggest that the hypoglycemic activity of Lf could be linked to the improvement of insulin resistance, which can be attributed to the attenuated activation of the corticosterone axis. A second study was carried out to rule out the effects of the intraperitoneal administration of bLf in rats before intravenous glucose injection (intravenous glucose-tolerance test, IVGTT) vs. OGTT, discounting the factor of glucose absorption from the small intestine. Interestingly, bLf did not affect any glycemic parameters in IVGTT, while it reduced plasma glucose and maintained insulin levels in OGTT. These latter effects were associated with lower levels of the plasma glucose-dependent insulinotropic polypeptide (GIP) and to an increase in total plasma glucagon-like peptide-1 (GLP-1) in the bLf-treated group compared with saline-treated controls [
92]. In addition, bLf administration was found to increase the absorption of glucose into the everted jejunum sac [
92]. All these observations support the idea that bLf can exert its hypoglycemic function by sustaining insulin secretion via GLP-1 upregulation while, at the same time, leaving glucose absorption from the intestine unaffected. Within this framework, one study suggests that Lf can directly contribute to the enhancement of glucose transport into small-intestinal epithelial cells by sodium-dependent glucose transporter (SGLT) 1, by downregulating the Ca
2+ and cAMP signaling pathways [
93]. A more recent study on a murine model of streptozotocin-induced type 2 diabetes mellitus (T2DM) reported the efficacy of bLf administration in decreasing serum concentrations of glycated serum protein and fasting insulin, and increasing liver insulin sensitivity, mainly through the upregulated expression of the insulin receptor (IR), insulin receptor substrate (IRS)-1, GLUT-4, phosphatidyl inositol 3-kinase (PI3K) and Akt. Moreover, bLf reversed the abnormal pancreatic islets of diabetic mice by reducing oxidative stress and inflammation responses, as evidenced by the downregulation of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β in the serum or liver [
94]. These latter results are in line with the sole in vivo study on type 2 diabetic pediatric patients, where camel Lf (cLf) was tested for its potential antidiabetic effect [
95]. Sixty young obese patients with type 2 diabetes treated with standard antidiabetic therapy were divided into two arms, one of which was orally administered with cLf capsules (250 mg/day, p.o) for 3 months. CLf treatment decreased diabetes-associated inflammation by inhibiting the Toll-like receptor (TLR)-4–NF-kB–SIRT-1 axis with a corresponding reduction in the serum pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Consequently, a significant increase in insulin expression and a concomitant decrease in serum glucose were found [
95]. This was the first study to point out an association between the Lf-induced hypoglycemic effect and an increase in peroxisome proliferator-activated receptor (PPAR)γ expression, suggesting that the antidiabetic effects are due to different features of Lf, from its anti-inflammatory properties to its ability to boost insulin signaling and its insulin-sensitizing efficacy through the PPARγ-dependent cascade. This latter mechanism is involved in glucose homeostasis by both upregulating the liver glucokinase, the rate-limiting enzyme of glycolysis, and stimulating the hepatic uptake of glucose. Overall, these data indicate that Lf supplementation may play an important role in counteracting a glucose imbalance, whether related or unrelated to type 2 diabetes (
Figure 1).
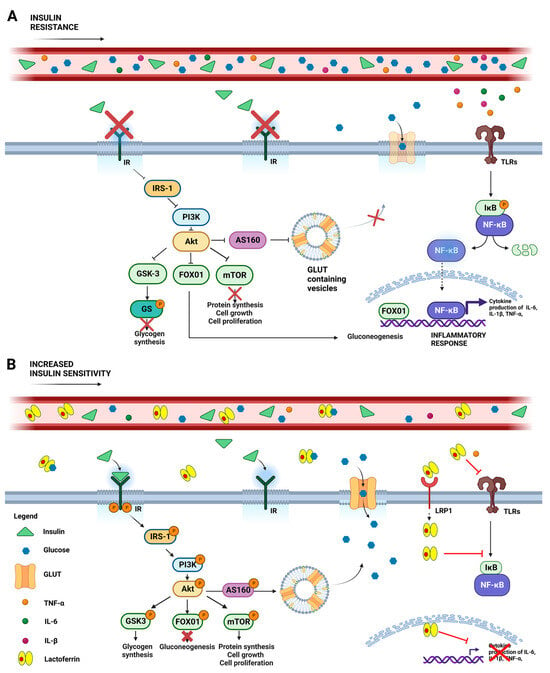
Figure 1. Schematic representation of systemic glycemic disorders in the absence (
A) or presence (
B) of lactoferrin. (
A) Insulin resistance is a clinical condition where insulin’s ability to promote glucose absorption and utilization is impaired, resulting in elevated blood glucose levels. This condition hampers IR autophosphorylation, which impairs the IRS-1/PI3-kinase/AKT pathway and results in aberrant downstream signals, including GSK3 activation and the consequent inhibition of glycogen synthesis via the phosphorylation of GS; the reduction of mTOR-mediated protein synthesis, cell growth and proliferation; as well as FOX01nuclear translocation, which promotes gluconeogenesis and the inflammatory response. Stimulation of TLRs by TNF-α, IL-6, and IL-1β exacerbates insulin resistance through the inflammatory IKβ/NF-κB pathway, increasing cytokine expression. (
B) Lactoferrin treatment counteracts these detrimental effects by boosting insulin binding to IR, thereby activating the IRS-1/PI3-kinase/Akt pathway. Akt activation results in AS160 phosphorylation, prompting GLUT to relocate from intracellular vesicles to the cell membrane, thus improving glucose uptake. Simultaneously, Akt-mediated FOX01 phosphorylation inactivates its nuclear translocation, while GSK3 and mTOR phosphorylation promote glycogen and protein synthesis, respectively. In addition, the protective effect of Lf could be explored by its ability to bind glucose and by virtue of its anti-inflammatory activity, thus counteracting TLR-mediated detrimental signaling. Abbreviations: insulin receptor (IR); insulin receptor substrate-1 (IRS-1); phosphatidyl inositol 3-kinase (PI3-Kinase); Akt, also known as protein kinase B; GSK3 (glycogen synthase kinase-3); GS (glycogen synthase); FOX01 (forkhead-box protein 01); Toll-like receptors (TLRs); tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6); interleukin-1β (IL-1β); nuclear factor kappa B (NF-κB); inhibitor of NF-κB (IKβ); glucose transporter (GLUT). Created with
BioRender.com (accessed on 15 June 2023).
3. Lactoferrin and Lipid Metabolism
Lipids are the energy reserves of animals and perform various functions, from acting as chemical messengers to the maintenance of body temperature and key constituents of cell membranes [
96,
97]. To enter the circulation, dietary fats are first emulsified via bile acids (BA) and then absorbed by enterocytes, where they are resynthesized and packed into lipoprotein particles. During their journey through the vascular system, nascent chylomicrons lose two minor apoproteins (ApoA-I and ApoA-IV) that are replaced by ApoE and ApoC-II, which are crucial for further chylomicron processing. In fact, ApoC-II activates adipocyte lipoprotein lipase (LPL), which facilitates the digestion of the chylomicron TG into fatty acids (FA) and glycerol, while ApoE is recognized by the hepatocyte LDL receptor, the LDL receptor-related protein (LRP), and scavenger receptor B-1, which facilitate the endocytic uptake of the chylomicron remnants [
98].
FA oxidation and de novo synthesis, as well as the expression of fatty acid transport proteins (FATPs), are closely regulated by various nuclear receptors, such as PPARα, PPARγ, and the bile acid receptor/farnesoid X receptor (FXR) [
99].
Glucose and lipid metabolism are linked in many ways. Insulin affects the de novo synthesis of lipids at multiple levels, via the induction of lipogenic genes, the activation of sterol regulatory element binding protein 1c (SREBP-1c), and the Akt-regulated production of very-low-density lipoproteins (VLDLs) [
99]. It is well known that diabetic patients often present with a typical dyslipidemia, characterized by elevated triglycerides, low HDL-C, and a predominance of small dense LDL particles. The existence of a link is further supported by the fact that insulin stimulates fatty acid synthase (FAS) expression via the PI3K pathway. At a transcriptional level, SREBP-1c and carbohydrate-responsive-element-binding protein (ChREBP), a glucose-dependent transcription factor, synergistically induce the expression of FAS and acetyl-CoA carboxylase (ACC) [
98].
The potential of Lf in rebalancing lipid dysmetabolism has been investigated in several studies using in vitro and animal models, with few clinical approaches. From the encouraging evidence on the application of whey proteins in this field, bLf was first demonstrated in the early 2000s to reduce plasma and hepatic triacylglycerol and non-esterified fatty acids (NEFA), with a parallel increase in HDL-C levels, in mice fed with a standard diet [
100]. Curiously, the same results were not recorded in mice fed with a high-fat diet (HFD), suggesting that Lf is only partially efficient. Similar results were obtained in a comparable study, where bLf intragastric administration was found to decrease lipid accumulation in the liver and mesenteric fat in mice [
101]. On the other hand, in a model of rat fed with a high-cholesterol diet (HCD), intragastric administration of bLf significantly ameliorated homocysteine and leptin levels and decreased serum LDL-C and total cholesterol, while increasing serum HDL-C, as evidenced by the up-regulation of ApoA-I expression [
102]. Similarly, the oral administration of enteric-coated bLf tablets significantly reduced total and LDL cholesterol levels in the serum, without interfering with HDL-C levels, in a high-fat and high-cholesterol diet model. Moreover, both LDLR and 3-hydroxy-3-methyl glutaryl CoA reductase (HMGCR) were significantly upregulated upon bLf treatment, and their transcriptional factor SREBP-2 also displayed a tendency toward upregulation [
103]. These studies have reaffirmed, once again, how crucial the choice of the experimental model is to verify/deny the effectiveness of a compound and the associated biological activity. In C57Bl/6J mice, Lf supplementation enhanced weight loss, suppressed weight re-gain and protected against the development of fatty liver formation, but it also ameliorated glucose tolerance and adipocyte tissue inflammation, without interfering with energy intake [
78]. Other animal studies have evidenced the protective role of oral bLf administration against the metabolic dysregulation associated with bacterial LPS [
104] and intestinal flora dysbiosis [
105,
106]. In these latter papers, bLf was found to be able to rebalance both glucose and lipid metabolic disorders and to restore inflammatory parameters, thus highlighting the multifaceted mechanism of action of the glycoprotein [
104,
105,
106]. Within the same framework, bLf was found to be an agonist of inulin, a prebiotic soluble fiber produced by many plants, in decreasing energy intake, body weight, fat and lean mass, along with the respiratory quotient, in diet-induced obese rats [
107]. Of note, Lf was also found to inhibit dietary cholesterol absorption, while increasing its fecal excretion via interactions with bile acids in rats [
108]. This effect was investigated in detail in C57BL/6J mice fed with high-fat/high-cholesterol diet containing cholate, where Lf was demonstrated to promote the expression of enzymes involved in both BA synthesis and conjugation, as well as to hinder hepatic cholesterol deposition through downregulation of the FXR-mediated enterohepatic axis [
109]. Interestingly, in ApoE
−/− mice, a model for studying cholesterol-induced atherosclerosis, Lf supplementation was able to revert the effect of a high-fat and high-cholesterol diet by significantly decreasing serum and hepatic cholesterol levels and the average lesion area in the whole aorta while increasing fecal cholesterol contents. These results were associated with the Lf-mediated downregulation of hepatic HMG-CoA reductase and the upregulation of cholesterol 7-alpha hydroxylase, two rate-limiting enzymes involved in the synthesis of cholesterol and bile acids, respectively [
109].
At a mechanistic level, Lf anti-adipogenic effects were investigated in several papers [
81,
110,
111,
112]. All these studies have demonstrated the ability of Lf to suppress the adipogenic differentiation of mouse pre-adipocyte MC3T3-G2/PA6 cells [
110], mouse embryonic 3T3-L1 cells [
90], and rat mesenteric fat-derived pre- and mature adipocytes [
111,
112]. Upon Lf treatment, in addition to the decreased levels of lipid droplets, CCAAT/enhancer-binding protein alpha (C/EBP-α) and PPARγ pathways were commonly found to be downregulated [
90,
110,
111,
112] along with other specific markers of adipogenesis, such as aP2 and adiponectin [
110], perilipin and the cAMP axis [
112], or acetyl-coenzyme A carboxylase alpha (ACC) and fatty acid synthase (FASN) via activation of the AMPK and Rb pathways [
90]. Proteomic analysis in mature adipocytes from primary cultures of rat mesenteric adipocytes confirmed the direct involvement of cAMP and extracellular signal-regulated kinase (ERK) pathways and the downstream activation of CREB in the Lf-induced activation of HSL, a key enzyme catalyzing the rate-limiting step of lipolysis via LRP1 [
113].
On the other hand, the proadipogenic effect of bLf was demonstrated in a study on human subcutaneous and visceral preadipocytes. HLf was found to activate proadipogenic genes, including PPARγ, ACC, FASN and adiponectin, and inhibit both AMPK/Rb signaling cascades and the pro-inflammatory cytokines interleukin-8 (IL-8), IL-6 and monocyte chemoattractant protein-1 (MCP1) [
114]. The proadipogenic role of Lf was also confirmed by the same group in a human adipocyte model of LTF knockdown, where the downregulation of endogenous Lf was associated with decreased adipogenic, lipogenic, and insulin-signaling-related gene expression and a significant increase in the gene expression of inflammatory mediators [
115].
These divergent results on the anti-/pro-adipogenic activity of Lf could be ascribed both to the source of Lf (bovine vs. human) and to the different cell models tested, as transformed cells could respond differently than primary cultures to external stimuli, including Lf [
9]. In reprogrammed human brown adipocytes generated using PLAT-GP cells, bLf addition promoted energy expenditure and oxygen consumption by upregulating uncoupling protein 1 (UCP1) expression through the cAMP-PKA signaling pathway via the LRP1 receptor [
116], thus reinforcing the proposal for the dietary application of Lf as a general booster of energy metabolism.
Nearly the same action mode and mechanistic processes were found to be exploited by Lf in animal models of dyslipidemia. In 2018, two studies reported the effects of orally administered Lf in high-fat diet-induced obese C57BL/6J mice [
117,
118]. Although both studies showed improvements, upon Lf treatment, in physical and serological parameters, such as visceral fat deposition, hepatic steatosis, lipid and glucose serum levels, along with a significant decrease in the expression of some lipogenic enzymes, such as SREBP-1, FAS. and ACC, differences in the activation of AMPK signaling were found. In line with in vitro models, Min and colleagues reported an increase in the p-AMPK/AMPK ratio following Lf treatment [
118], but no significant difference in p-AMPK/AMPK and PPARα levels were found between HFD and HFD + Lf groups in the study by Xiong and colleagues [
117]. In the latter study, apart from the significant downregulation of MCP-1, a chemokine involved in the progression of hepatic inflammation and fibrosis, Lf treatment was not found to be able to induce a significant decrease in mRNA levels of both IL-6 and TNF-α, two cytokines strictly interconnected with the AMPK cascade that was shown to be able to inhibit TNF-α-stimulated IKK/NF-κB and IL-6-induced JAK/STAT3 signaling pathways [
119]. Altogether, these data could suggest that Lf may regulate hepatic lipid metabolism via pathways other than the AMPK signaling cascade; however, more efforts are required to better test and prove this hypothesis.
A recent metabolomic study was carried out to investigate the putative beneficial effects of early-life Lf administration in newborn piglets [
120]. Pathway analysis of the metabolomic profiles showed that the Lf treatment affected lipid and amino acid metabolism in the liver, with a decrease in plasma urea nitrogen and increased levels of plasma albumin. In addition, Lf-treated piglets showed upregulated expression of β-oxidation-related gene (CPT1) and hepatic TCA and increased phosphorylation levels of mTOR and p70S6 kinase as well as increased levels of proteolytic enzymes and amino acid transporters in the jejunum. Further, Lf treatment decreased malondialdehyde levels and increased levels of hepatic antioxidant glutathione (GSH), glutathione peroxidase (GSH-Px), and glutamate cysteine ligase catalytic subunit (GCLC) [
120], thus primarily evidencing positive effects of Lf consumption in the modulation of metabolic and defense pathways during early life.
Finally, a double-blind, placebo-controlled design study carried out in humans showed a potent anti-obesity effect of enteric-coated bLf tablets (300 mg/day) in decreasing visceral fat accumulation after an 8-week administration to Japanese men and women with abdominal obesity. Of importance, no adverse effects regarding blood lipid or biochemical parameters were recorded in the bLf-treated group [
121], thus highlighting, once again, the global safety of this molecule and encouraging its application as a promising agent for the control of visceral fat accumulation (
Figure 2).
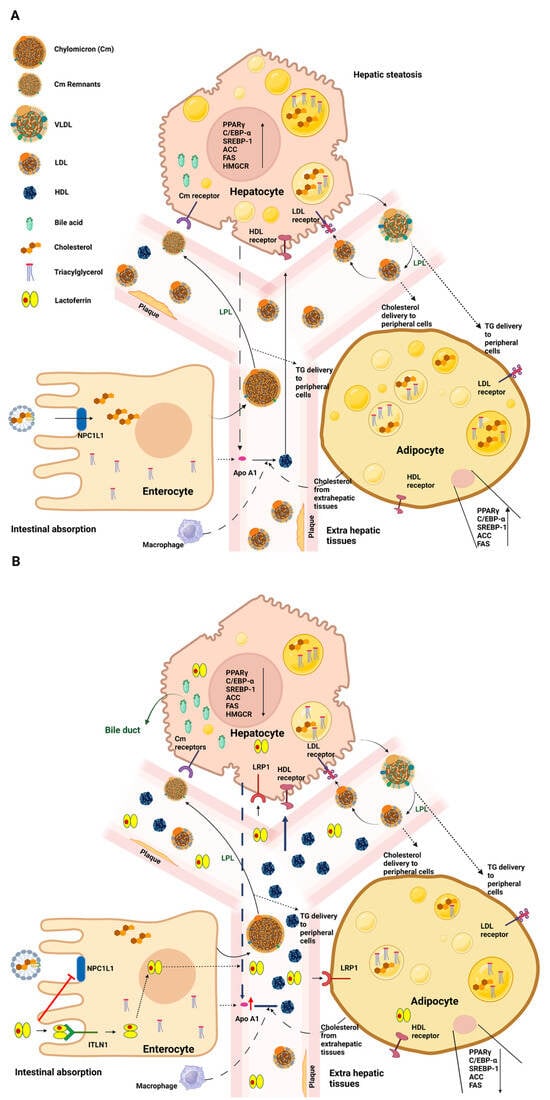
Figure 2. Schematic representation of systemic lipidic disorders in the absence (
A) or presence (
B) of lactoferrin. (
A) Dietary fats, after emulsification and hydrolysis by bile acids, are taken up by enterocytes. Luminal cholesterol is transported across the brush border via NPC1L1 and, together with other lipids, is packed into nascent Cm particles. Once in the bloodstream, the Cm particles undergo transformations and are subject to the influence of LPL, which assists in converting Cm triglycerides into fatty acids and glycerol. Subsequently, hepatocytes internalize the Cm remnants through Cm receptors. In the context of systemic lipid disorders, upregulation of PPARγ, C/EBP-α, and SREBP-1 pathways and of other adipogenesis markers, such as ACC, FAS, and HMGCR, occurs, along with an increase in the levels of intracellular lipid droplets. Hepatic cells release abnormal quantities of VLDL, which are converted to LDL by LPL to release triglycerides to various tissues. This process elevates circulating LDL levels, contributing to the development of atherosclerotic plaques. On the other hand, low levels of circulating HDL are recorded. (
B) Upon oral Lf treatment, a reduction in cholesterol absorption in the intestine is observed. In accordance with the downregulation of C/EBP-α, PPARγ, and SREBP-1pathways and the reduction of specific lipogenic enzymes, including FAS and ACC, a decrease in lipid droplet levels and circulating LDL is found. Interestingly, higher serum levels of HDL are observed. See text for further details. Abbreviations: Neiman–Pick C1-like 1 (NPC1L1); chylomicron (Cm); lipoprotein lipase (LPL); peroxisome proliferator-activated receptor γ (PPARγ); CCAAT/enhancer-binding protein (C/EBP-α); sterol-regulatory-element-binding protein-1 (SREBP-1); acetyl-CoA carboxylase (ACC); fatty-acid synthase (FAS); 3-hydroxy-3-methyl glutaryl CoA reductase (HMGCR), very-low-density lipoprotein (VLDL); low-density lipoprotein (LDL); high-density lipoprotein (HDL). Created with
BioRender.com (accessed on 15 June 2023).
This entry is adapted from the peer-reviewed paper 10.3390/ijms242115925