Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The most prevalent gynecologic cancer is endometrial cancer. In 2018, there were 121,600 new cases of this cancer diagnosed in Europe, roughly 6.6% of all malignancies found in women. A total of 29,600 gynecologic-cancer-related deaths are predicted to occur each year, accounting for over 3.5% of all cancer-related deaths.
- endometrial carcinoma
- metastasis
- long-term survival
1. Evidence on Cutaneous Metastasis of Endometrial Cancer
researchers found eight case report studies of cutaneous metastasis of endometrial cancer. The cohorts included patients between 52 and 73 years old. The histological type of the metastasis was endometroid adenocarcinoma in six cases, carcinosarcoma in one case, and leiomyosarcoma in another case. The time from the diagnosis of endometrial cancer to the appearance of cutaneous metastasis was between 2 months and 3 years (Table 1).
Table 1. Summary of authors, year of publishing, study type, age of included patients, histological findings of primary disease, time from first diagnosis to the appearance of cutaneous metastasis, operation performed upon primary diagnosis of endometrial cancer, and the location of cutaneous metastasis.
| Authors | Year | Study Type | Age | Histological Findings | Time from First Diagnosis to Cutaneous Metastasis | Initial Operative Therapy | Location of Metastasis |
|---|---|---|---|---|---|---|---|
| Atallah et al. [1] | 2014 | CR | 62 | E.A. | 3 Years | Total hysterectomy, bilateral salpingho-oopherectomy, and pelvic lymphadenectomy | Located at the site of thelaparotomy extending down to the vulva |
| Clairwood, MD [2] | 2016 | CR | 57 | Carcinosarcoma | 6 Months | Total hysterectomy, bilateral salpingho-oopherectomy, and pelvic and paraaortic lymphadenectomy | Asymptomatic noduleslocated on her left lower cheek, back, and flank |
| Giovanna Giordano [3] | 2005 | CR | 66 | E.A. | 8 Months | Total hysterectomy, bilateral salpingo-oophorectomy, and selective pelvic lymph node sampling | Vulvar posterior commissure |
| Nikolaos Barbetakis [4] | 2009 | CR | 52 | Leiomyosarcoma | 2 years | Total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy | Cutaneous skull metastasis |
| Baydar [5] | 2005 | CR | 58 | E.A. | 19 Months | No information specified | Cutaneous metastasis ofinitial operation site |
| Fatima Zahra El M’rabet [6] | 2012 | CR | 72 | E.A. | 6 Months | Total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and paraaortic lymph node sampling | Subcutaneous nodules located on her trunk, extremities, and scalp |
| Yuan Fan [7] | 2021 | CR | 54 | E.A. | 22 Months | No information specified | Cutaneous breast metastasis |
| Stonard MC [8] | 2003 | CR | 73 | E.A. | 2 Months | Total abdominal hysterectomy, bilateral salpingo-oophorectomy | Skin lump on her leftlower leg |
| Nienhaus | 2023 | CR | 51 | E.A. | At primary diagnosis | Hysterectomy/abdominal adnexectomy with excision of the navel, pelvic and paraaortic lymphonodectomy | Cutaneous, umbilical metastasis |
The location of cutaneous metastasis varied. In two studies, a metastasis was located on the skull and two studies showed metastasis on the vulva. Metastases were also located on the navel, trunk, breasts, flank, and lower leg. The initial operation performed for endometrial cancer depended on the FIGO stage, with total abdominal hysterectomy and bilateral salpingo-oophorectomy with or without a pelvic or para-aortic lymphadenectomy. Some patients only underwent lymph node sampling. When a lymphadenectomy was performed, in most cases, metastasis in the lymph nodes was observed (Table 1).
In four of the studies, the initial FIGO stage was I; in three others, the FIGO state was III; and in one case, the FIGO stage was IVB with spread of the disease to the pelvic lymph nodes (Table 2).
Table 2. Summary of study: authors, year of publication, study type, age of included patients, FIGO stage at primary diagnosis, therapy performed after surgery at primary diagnosis, treatment of metastatic disease, and time until death after diagnosis of cutaneous metastasis.
| Authors | Year | Study Type | FIGO Stage at First Diagnosis | Therapy after Initial Diagnosis | Treatment of Metastatic Disease | Time until Death after Diagnosis of Cutaneous Metastasis. |
|---|---|---|---|---|---|---|
| Atallah et al. [1] | 2014 | CR | I B | Extern pelvis radiation | Four cycles of chemotherapy with etoposide and cisplatin | 5 months after chemotherapy |
| Clairwood, MD [2] | 2016 | CR | III C1 | Carboplatin/taxol and whole pelvis radiation | Radiotherapy | 3 months |
| Giovanna Giordano [3] | 2005 | CR | III C | None, due to fragility | Hormone therapy | 14 months |
| Nikolaos Barbetakis [4] | 2009 | CR | FIGO III | CTX, RTX | Operation, chemotherapy | Still alive after 8 months of therapy (at the time of article presentation) |
| Baydar [5] | 2005 | CR | - | RTX, CTX | Combination chemotherapy | After two cycles of chemotherapy |
| Fatima Zahra El M’rabet [6] |
2012 | CR | FIGO IC | N | None due to poor general condition | 2 weeks |
| Yuan Fan [7] | 2021 | CR | FIGO IB | None | Chemotherapy, immunotherapy, and hormone therapy | 7 months |
| Stonard MC [8] | 2003 | CR | FIGO IC | None | No information | No information |
| Nienhaus | 2023 | CR | FIGO IVB | RTX | Chemotherapy, immunotherapy, and hormone therapy | 13 years |
researchers can observe that after the appearance of cutaneous metastasis, the overall survival rate is poor, at between 2 weeks and 14 months. In this case, there was a long overall survival of over 13 years. The therapy had no significant effect on the overall survival rate. Chemotherapy was performed in five studies and radiotherapy in two cases. Hormone therapy was performed in three cases, and, in one case, no therapy was performed due to the poor general condition of the patient. The appearance of cutaneous metastasis was never the only sign of metastasis. In most cases, there were also signs of pulmonary, intraabdominal, and lymph node metastasis and pleural effusion (Table 2).
2. Case Presentation
researchers present the case of a 51-year-old woman presented to a hospital in Bochum, Germany, with a diagnosis of endometrial adenocarcinoma pT1b pN1(4/24) M1 (cutaneous, umbilical metastasis) G1-2 L1 R0 in 2008. A hysterectomy/abdominal adnexectomy with excisions of the navel, pelvic, and para-aortic lymphadenectomies was performed. She received after loading radiation three times.
The patient had no medical history except for arterial hypertension. In the family, there was an aunt who received a breast cancer diagnosis at the age of 56 and also a cousin who received a breast cancer diagnosis at the age of 50. Both are maternal relatives.
She returned to Bochum in June 2018 with the following symptoms: on the right ventral chest wall, she had a large tumor with a perforation in the pericardium of approximately 15 × 20 cm, an abdominal inguinal tumor on the right, and an ulcerating tumor on the lower left side of the abdomen. A computed tomography of the thorax and abdomen was performed in 09/2018 (Figure 1 and Figure 2) and a magnetic resonance imaging of the thorax was performed in 06/2018 (Figure 3 and Figure 4).
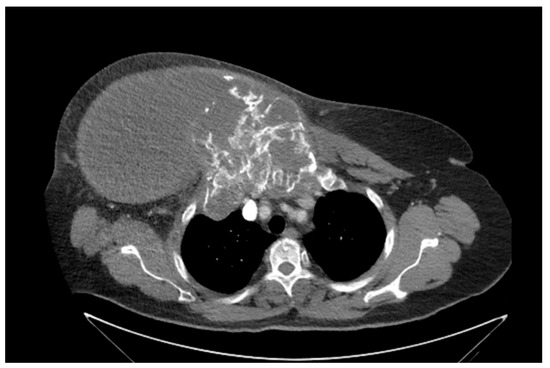
Figure 1. Computed tomography of thorax and abdomen (CT Thx/Abd) in 2018. Researchers detected a large tumor on the right anterior thorax with calcifications and invasion in the mediastinum.
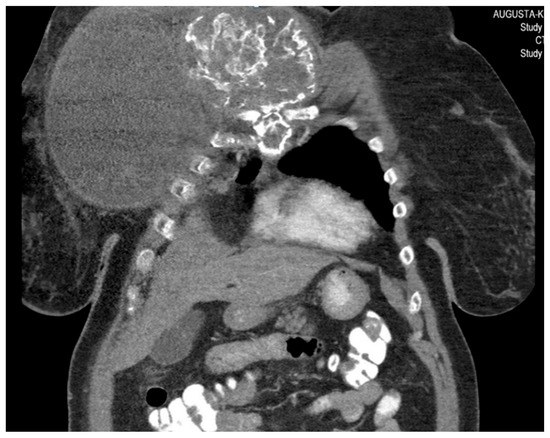
Figure 2. Computed tomography of the thorax and abdomen (CT Thx/Abd) in 2018. There is a large, partially calcified soft tissue structure of the right breast with central hypodensity and possibly mucinous parts. The image shows infiltration of the thoracic wall and transfer to the mediastinum, with destruction of the corpus and manubrium sterni and infiltration of the musculus pecotralis major and the musculus serratus anterior on the right. Additionally, destruction of the adjacent medial clavicle on the right and of the first rib medial on the right was observed. The formation directly infiltrates the mediastinum, with infiltration of the pericardium and direct contact with the aortic arch. The maximum extent of this conglomerate was 25.2 × 14.8 × 10.0 cm (VU 22.7 × 16.6 cm axial; not fully recorded cranially). There was no flow obstruction of the right-side vessels of the upper thoracic aperture.
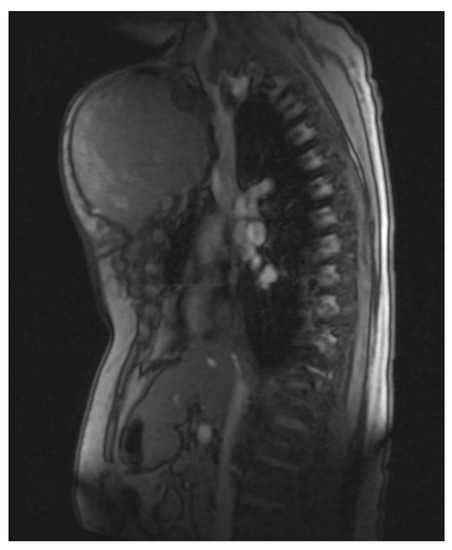
Figure 3. Magnetic resonance imaging (MRI 2018) showing the large tumorous masses on the anterior thoracic wall, infiltration of the mediastinum per continuitatem, and contact with the aorta.
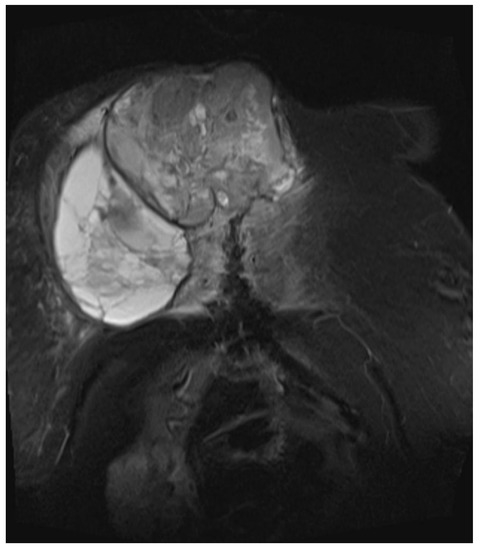
Figure 4. Magnetic resonance imaging (MRI 2018) showing the bis soft tissue structure, partially calcified and infiltrating the sternum.
The histopathological findings (Figure 5) of a probe of the large chest and inguinal tumors show an endometroid adenocarcinoma, G2, with expression of cytokeratins MNF116 and CK7. The reaction to CK20, CDX2, Gata3, and TTFI was negative. The PAX8 marker was positive. The receptor status was positive to estrogen 90% and progesterone 40%. PDL1 was 1%. The intrinsic subtype was a microsatellite stabile tumor.
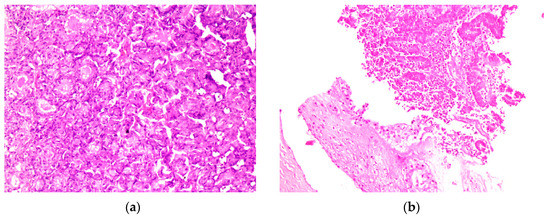
Figure 5. (a) HE; 250× magnification under a microscope, metastasis of the rectus abdominis muscle; (b) HE 250× magnification under a microscope, invasion of the soft tissue from the endometrial cancer metastasis on the right thorax wall. Abbreviations: HE, hematoxylin -eosin.
Chemotherapy with carboplatin/paclitaxel cycle I was initiated on 19 June 2018 and radiotherapy of the abdominal tumor mass, the inguinal tumor on the right side, and the ulcerating tumor on the lower left side of the abdomen was performed between 6 June 2018 and 8 August 2018. The dose of radiation was 50.4 Gy/1.4 Gy. On 15 August 2018, carboplatin/paclitaxelis was administered weekly and the patient had a reaction to the contrast agent under RT RCXT. In 2018/09, researchers tried to determine the cause of the prolonged pancytopenia that led to the limitation of therapy and thrombocytopenia. The most likely origin of toxicity seemed to be related to the drugs administered after cytostatic therapy. A differential diagnosis of idiopathic thrombocytopenia was not the most likely. A bone marrow diagnosis does not currently indicate myelodysplasia. Between 2018/11 and 05/2019, researchers switched to megestrol acetate therapy. The treatment was interrupted because the medicine was not available. In 2019/05, thrombosis of the left common iliac vein occurred. Apixaban therapy was initiated in 2019/07. An abdominal CT scan was performed, and modest progress was noted. Treatment with tamoxifen 20 mg/day was initiated. An abdominal ultrasound was performed in 2019/09 and stable disease was confirmed; thus, tamoxifen treatment was continued. On 10 June 2020 MB, progression of the chest disease with a pronounced volume and appearance of skin metastasis were noted. A comprehensive molecular diagnosis showed possible skin metastasis. Therapy with pembrolizumab (no carboplatin and pemetrexed due to limited hematological competence) was initiated. In 2020/09, CT was performed; the results showed new progression and progressive intramammary manifestation of parts of the tumor, which was now spreading to the skin of the right breast. Pembrolizumab therapy was then initiated on 23 September 2020. Several symptoms were suspected as hypertension. In 2019/04, thrombosis of the left common iliac vein was noted; apixaban therapy was initiated. There was a discrepancy between the manifestation of the tumor and the excellent clinical status to date (extremely slow-growing tumor).
The patient developed axillary skin metastasis of the right axilla in 2021 (Figure 6). Intensity-modulated radiotherapy(IMRT/RapidARc) as a palliative therapy with 30/2 Gy and simultaneous boost with 7.50 Gray was performed. The cumulated dose was 37.5 Gray, applied to the right axilla. The patient had no adverse reactions to radiotherapy. Computed tomography of the thorax and abdomen in 01/2021 (Figure 7 and Figure 8) showed the slow growth of the masses compared to 2020.
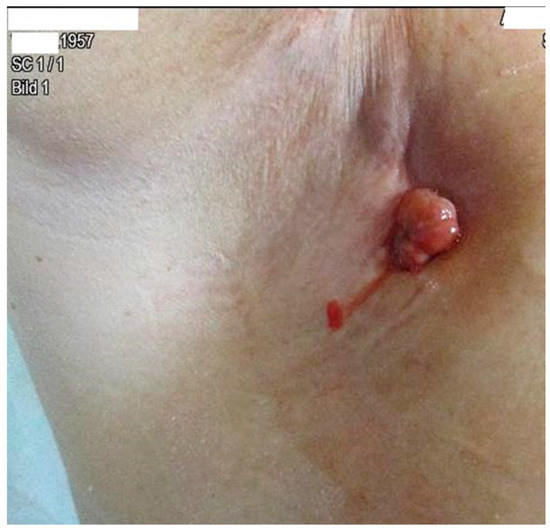
Figure 6. Image of axillary cutaneous metastasis in the right axilla in January 2021.
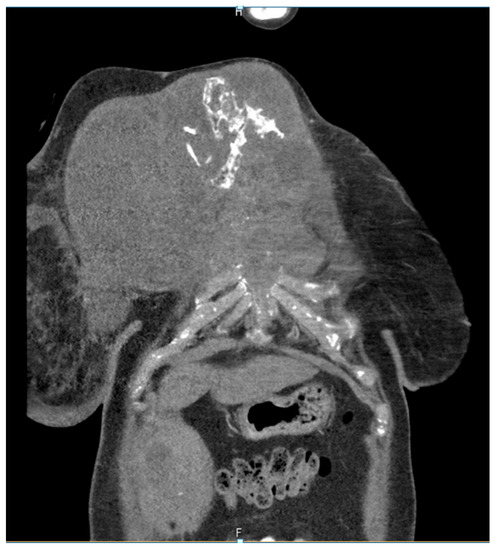
Figure 7. Computed tomography of the thorax and abdomen (CT Thx/Abd) in 2021showing large tumorous masses on the right anterior thoracic wall and right mammary gland with extensive calcification. This extended transversally over approximately 13.4 × 24.9 cm compared to approximately 12.7 × 25.4 cm in the CT scan on 04/20. The tumor formations extend craniocaudally over approximately 17.6 cm, compared to up to approximately 16.1 cm in 04/20 and up to approximately 17.0 cm in 09/20. Includes osteosynthesis material at the HWK 5/6 level.
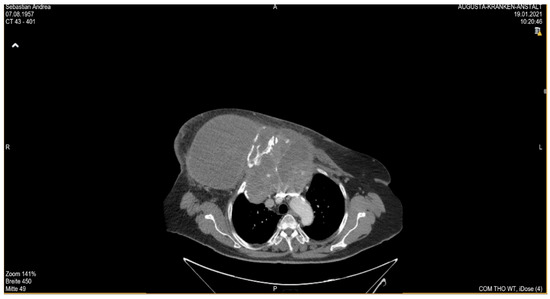
Figure 8. Computed tomography of the thorax and abdomen (CT Thx Abd) in 2021 showing the continued spread in the cervicothoracic junction. Osteolytic destruction of the medial right clavicle, the first to third right ventral ribs, the manubrium sterni and corpus sterni, as well as the first and second ribs on the left was observed. Infiltration of the caudal costal cartilage transitions was also observed.
The patient passed away in 2021.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13152603
References
- Atallah, D.; el Kassis, N.; Lutfallah, F.; Safi, J.; Salameh, C.; Nadiri, S.; Bejjani, L. Cutaneous metastasis in endometrial cancer: Once in a blue moon—Case report. World J. Surg. Oncol. 2014, 12, 86.
- Clairwood, M.; Yasuda, M.; Belazarian, L.; Deng, A. Unusual Cutaneous Metastasis of Uterine Carcinosarcoma: A Case Report and Review of the Literature. Am. J. Dermatopathol. 2016, 38, 366–369.
- Giordano, G.; Gnetti, L.; Melpignano, M. Endometrial carcinoma metastatic to the vulva: A case report and review of the literature. Pathol. Res. Pract. 2005, 201, 751–756.
- Barbetakis, N.; Paliouras, D.; Asteriou, C.; Samanidis, G.; Kleontas, A.; Anestakis, D.; Kaplanis, K.; Tsilikas, C. Cutaneous skull metastasis from uterine leiomyosarcoma: A case report. World J. Surg. Oncol. 2009, 7, 45.
- Baydar, M.; Dikilitas, M.; Sevinc, A.; Senel, S.; Senel, F.; Aydogdu, I. Cutaneous metastasis of endometrial carcinoma with hemorrhagic nodules and papules. Eur. J. Gynaecol. Oncol. 2005, 26, 464–465.
- M’rabet, F.Z.Z.E.; Hottinger, A.; George, A.C.; Tille, J.C.; Mesbahi, O.E.; Castiglione-Gertsch, M.; Bodmer, A. Cutaneous Metastasis of Endometrial Carcinoma: A Case Report and Literature Review. J. Clin. Gynecol. Obstet. 2012, 1, 19–23.
- Fan, Y.; Deng, H.; Tian, L.; Wang, J. Cutaneous metastases of endometrial carcinoma: Report of an unusual case and literature review. Arch. Gynecol. Obstet. 2021, 304, 1127–1133.
- Stonard, C.M.; Manek, S. Cutaneous metastasis from an endometrial carcinoma: A case history and review of the literature. Histopathology 2003, 43, 201–203.
This entry is offline, you can click here to edit this entry!
