Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As time has passed following the COVID-19 pandemic, individuals infected with SARS-CoV-2 have gradually exhibited a variety of symptoms associated with long COVID in the postacute phase of infection. Simultaneously, in many countries worldwide, the process of population aging has been accelerating.
- long COVID
- elderly
- COVID-19
- immunosenescence
1. Introduction
As of 26 October 2023, the World Health Organization (WHO) has reported 771,549,718 confirmed cases of COVID-19 worldwide since the outbreak of SARS-CoV-2 in February 2020 [1]. COVID-19 not only causes a series of acute pathological responses in the human body [2] but has also gained attention due to its postinfection sequelae, commonly referred to as long COVID or postacute COVID-19 syndrome. Scientists have been studying long COVID since April 2020, as seen in the LitCovid topic “Long COVID” on PubMed [3][4].
Long COVID is “a condition that occurs in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, typically occurring three months after the onset of COVID-19 symptoms, and lasting for at least two months without any other diagnosis to explain it. Common symptoms include fatigue, shortness of breath, cognitive dysfunction, and others; moreover, it generally affects daily functioning” [5]. The National Institute for Health and Care Excellence (NICE) defines it as “signs and symptoms that develop during or after an infection consistent with COVID-19 which continue for more than 12 weeks and are not explained by an alternative diagnosis” [6]. In a meta-analysis that included 15 studies and 47,910 patients [7], Lopez-Leon et al. reported that long COVID may manifest with as many as 50 or more different clinical symptoms. Among these symptoms, the most common were fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), dyspnea (24%), cough (19%), joint pain (19%), anxiety (13%), digestive disorders (12%), pain (11%), renal failure (1%), and PTSD (1%). Long COVID symptoms affect multiple organs and systems within the human body.
According to current research [8], the prevalence of long COVID is estimated to be between 31% and 69%, indicating that over 200 million individuals worldwide may experience long COVID symptoms. While some studies have suggested that older adults may not be at a higher risk of long COVID than younger individuals [9], this may be because there are more younger COVID-19 survivors, and the epidemiological statistics for long COVID in elderly people exclude a substantial number of fatalities and may introduce bias towards older adults [10]. Additionally, many mechanisms of long COVID remain unclear, and there is a lack of targeted and effective treatment options [11]. Considering that older adults are a population that requires considerable health care resources, long COVID in this population remains a major challenge in public health, clinical medicine, and basic medical research.
2. Characteristics of Older Adults Infected with COVID-19
2.1. Common Symptoms of COVID-19 in Elderly People
Currently, substantial research from around the world indicates that age is a significant risk factor for severe COVID-19 and adverse health outcomes [12]. In elderly individuals, common clinical symptoms of COVID-19 following SARS-CoV-2 infection include cough, fever, shortness of breath, dyspnea, myalgia, anxiety, depression, anosmia, and ageusia. Additionally, atypical symptoms such as neurological manifestations, elevated white blood cell counts, and elevated muscle enzyme levels are more prevalent among elderly individuals. Furthermore, as age increases, the probability of adverse clinical outcomes also escalates [13]. Undoubtedly, aging is an important risk factor for severe COVID-19 and its adverse health consequences, including hospitalization, ICU admission, and mortality [12].
2.2. Prolonged Viral Shedding
Since the outbreak of SARS-CoV-2, several reports have highlighted that the risks of hospitalization, ICU admission, and death increase with age [14][15][16][17][18], indicating that older adults were a high-risk group during the COVID-19 pandemic. During the acute phase of COVID-19, older adults exhibit distinct characteristics compared to younger age groups. The transmission of the virus depends on the shedding of infectious viral particles, which is influenced by both viral and host factors [19]. According to Chen et al., the median duration of SARS-CoV-2 RNA shedding is generally approximately 12 days, and as age increases, the median duration of viral shedding also increases. The median durations for patients under 16 years, 16–49 years, 50–64 years, and over 65 years old were 8.0 (6.0–15.0), 11.0 (7.0–15.0), 13.0 (10.0–17.0), and 13.0 (10.0–19.0) days, respectively. Advanced age is independently associated with prolonged shedding of SARS-CoV-2 RNA in the respiratory tract [20]. Although different studies report varying median times of viral RNA shedding, all demonstrate an association between older age and prolonged shedding of the virus [21][22][23]. Some studies have indicated that older patients (over 60 years) have a significantly higher proportion of continued positive SARS-CoV-2 detection in viral genome testing three weeks after initial infection compared to younger patients [24].
2.3. Intensive Tissue Damage Related to Inflammation in the Lung and Other Organs
In terms of medical imaging, there are also age-related differences among COVID-19 patients. In a retrospective analysis of chest CT images from 72 COVID-19 patients, older patients had a higher proportion of extensive lung lobe involvement and were more likely to have subpleural lines and pleural thickening [25]. Another retrospective analysis of chest CT images from 50 patients revealed that interlobular septal thickening and honeycombing were more common in older individuals. Moreover, there was a statistically significant difference in the total CT score among different age groups, with the average total score being 7.3 in the older group, higher than the 3.9 in the younger group [26]. Notably, both short-term and long-term follow-up of hospitalized and recovered COVID-19 patients showed that older age was a risk factor for pulmonary fibrotic lesions [27][28]. Additionally, older adults exhibit a decreased type I interferon (IFN-I) response and increased levels of proinflammatory cytokines such as interleukin (IL)-6, IL-12, IL-1β, and tumor necrosis factor (TNF)-α after SARS-CoV-2 infection, which may exacerbate the severity of COVID-19 in this population [29] (see Figure 1).
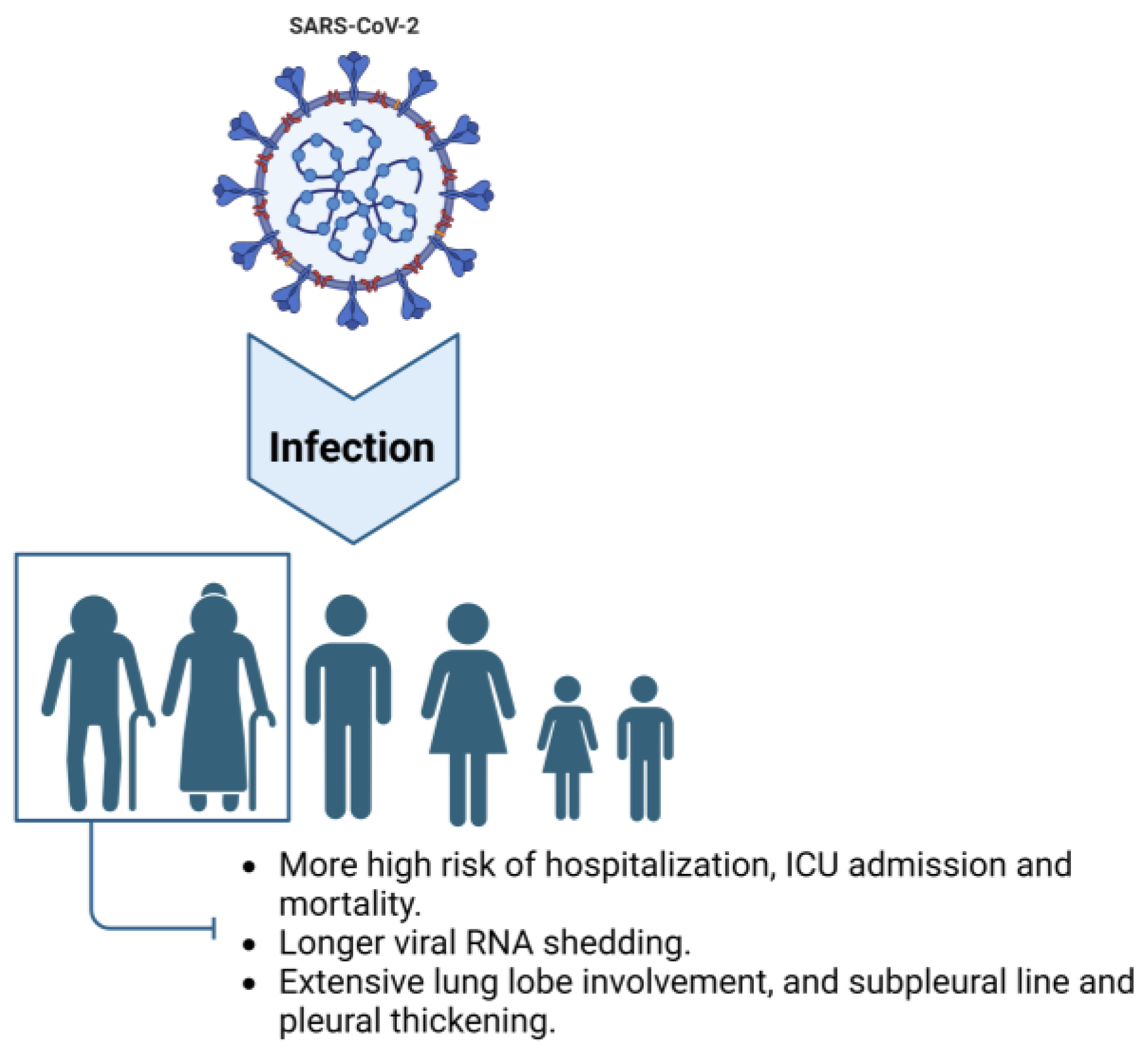
Figure 1. Characteristics of older adults infected with COVID-19. Compared to other age groups, elderly individuals exhibit a higher risk of hospitalization and ICU admission and a higher mortality rate when infected with SARS-CoV-2. Additionally, older adults experience longer viral shedding periods and display radiological symptoms characterized by extensive involvement of lung lobes, subpleural lines, and pleural thickening.
The age-related characteristics of SARS-CoV-2 infection have also been demonstrated in experimental animal models. Studies have found that older ferrets exhibited more severe lung pathology and clinical symptoms, while older non-human primates exhibited sustained proinflammatory responses [30]. In non-human primates of advanced age, Speranza et al., Blair et al., and Zheng et al. all noted that cytokine levels emphasized sustained proinflammatory responses [31][32][33]. Older rhesus macaques have also been found to have more active viral replication in the lungs and experience more severe interstitial pneumonia caused by the virus than younger macaques [34].
3. Risk Factors for Developing Long COVID in Elderly Individuals
SARS-CoV-2 invades the human body and causes pathological damage in four stages: (1) invasion; (2) blockade of antiviral innate immunity; (3) interaction between viral defense mechanisms and adaptive immunity; and (4) acute or long-term complications of COVID-19 [35]. These stages highlight the importance of the immune system in SARS-CoV-2 infection, and studies have shown that the severity and mortality of the infection in older adults is closely related to the characteristics of their immune system. In young and middle-aged people, the immune system is, normally, resting but able to mount a strong but transient dynamic response promptly after detecting an “invasion”. However, during the aging process, the immune system undergoes mild, chronic activation that leads to a prolonged response time and decreased response magnitude when stimulated [36]. This state, known as immunosenescence, is characterized by chronic low-grade inflammation and a decline in the ability to respond to and defend against external threats [37][38]. As a result, older individuals often experience worsening symptoms with increasing age, atypical clinical manifestations, and a delayed fever response when encountering SARS-CoV-2 infection.
3.1. Immunosenescence
The immune system plays a critical role in the response to SARS-CoV-2 infection, and the characteristics of the aging immune system are closely related to the increased risk of severe illness and mortality among older individuals. Aging is associated with immunosenescence, leading to a prolonged and less effective immune response, which may contribute to the more severe outcomes observed in elderly individuals infected with SARS-CoV-2.
Immunosenescence refers to changes in immune function caused by aging [39]. It not only substantially impacts both innate and adaptive immunity but is also a risk factor for most age-related diseases [40][41][42]. In the context of innate immunity, immunosenescence is characterized by decreased phagocytic capacity of neutrophils [43], reduced microbicidal activity [44], decreased number of macrophage precursors, reduced phagocytic function [45], diminished phagocytic capacity of monocytes [46], impaired production of new natural killer (NK) cells [47][48], and decreased cytotoxicity of NK cells [49]. In terms of adaptive immunity, aging is associated with decreased production of naive B cells and a reduced ability to respond to new antigens and leads to a shorter duration of IgG production by plasma cells and compromised specific humoral immune function against pathogens and vaccines [50][51][52]. Additionally, with the atrophy and functional decline of the thymus in older individuals, there is a decline in the number of naive T cells [53], while the number of antigen-specific memory T cells and effector T cells increases with age [54]. Research has shown that many cytokines, such as IFN-γ, IL-2, and TNF-α, in CD8+ cells increase with age, and IL-4, IL-6, and IL-10 increase in the memory subset [55]. Aging also affects the quantity, subset distribution, and function of Tregs [56] and leads to dysregulation of microRNAs (miRNAs) [36], which are non-coding single-stranded RNAs (ssRNAs) that play a crucial role in immune regulation [57]. In summary, with a series of immune dysfunctions, some functions are downregulated, while others are upregulated, leading to the occurrence of widespread low-grade chronic inflammation known as inflammaging [58].
3.2. Chronic Inflammation (Inflammaging)
Inflammation, responding to exogenous or endogenous infections or injuries, is regulated by the immune system. When the body perceives a threat, inflammation regulated by the immune system will be activated, which subsides when the threat diminishes [59][60]. However, older individuals, due to a prolonged lifetime of exposure to various stimuli, such as chronic infections, obesity, cellular senescence, and aggregation of exogenous or endogenous macromolecules in the body [61][62], combined with immunosenescence that occurs with aging, have an elevated level of age-related proinflammatory markers even in the absence of overt clinical disease or threats. This leads to a chronic low-grade inflammatory status known as “inflammaging” [63]. Such inflammaging is a risk factor for several age-related diseases, including but not limited to hypertension [64][65][66], type 2 diabetes [64], cardiovascular diseases (CVDs) [67][68], chronic kidney disease [69], cancer [70][71], and depression [72][73].
3.3. Comorbidity
Comorbidity is not only a risk factor for susceptibility to SARS-CoV-2 infection [74] but also a risk factor for the severity of COVID-19 [75]. In a study of 1590 laboratory-confirmed COVID-19 patients in China, the risk ratio for patients with at least one comorbidity was 1.79 (95% CI, 1.16–2.77), and for patients with two or more comorbidities, the risk ratio was 2.59 (95% CI, 1.61–4.17) [76]. Another meta-analysis including six studies and 1527 patients indicated that 17.1% of COVID-19 patients had hypertension, 16.4% had cardiovascular diseases, and 9.7% had diabetes [77]. Comorbidities are even more common in patients with severe COVID-19, including hypertension, diabetes, COPD, cardiovascular diseases, chronic kidney disease, malignancies, and others, which can lead to severe outcomes in infected individuals [75][78][79][80][81]. Hypertension, cardiovascular disease, and diabetes were the most common comorbidities in patients who died due to COVID-19 [82]. A meta-analysis of 51 studies involving 48,317 diagnosed COVID-19 patients indicated that the prevalence of hypertension, diabetes, and cardiovascular diseases was lower among young patients than among older patients, and hypertension, diabetes, and cardiovascular diseases were significantly associated with patient mortality across all age groups [83].
In summary, a cascade of events occurs in older individuals: immunosenescence leads to immune dysregulation, which results in inflammaging. This inflammaging contributes to the development of comorbidities in older individuals, increasing their vulnerability to SARS-CoV-2 infection and the severity of COVID-19 (see Figure 2). Furthermore, a higher number of SARS-CoV-2 infections can potentially increase the risk of long COVID in older individuals [84]. Additionally, the severity of COVID-19 increases the risk of long COVID. Retrospective analysis has shown that older age and severe illness are associated with a higher risk of fatigue and experiencing multiple long COVID symptoms [85]. Therefore, older adults not only face a higher risk during acute COVID-19 but also remain at a greater risk for long COVID, which can cause them physical and mental harm.
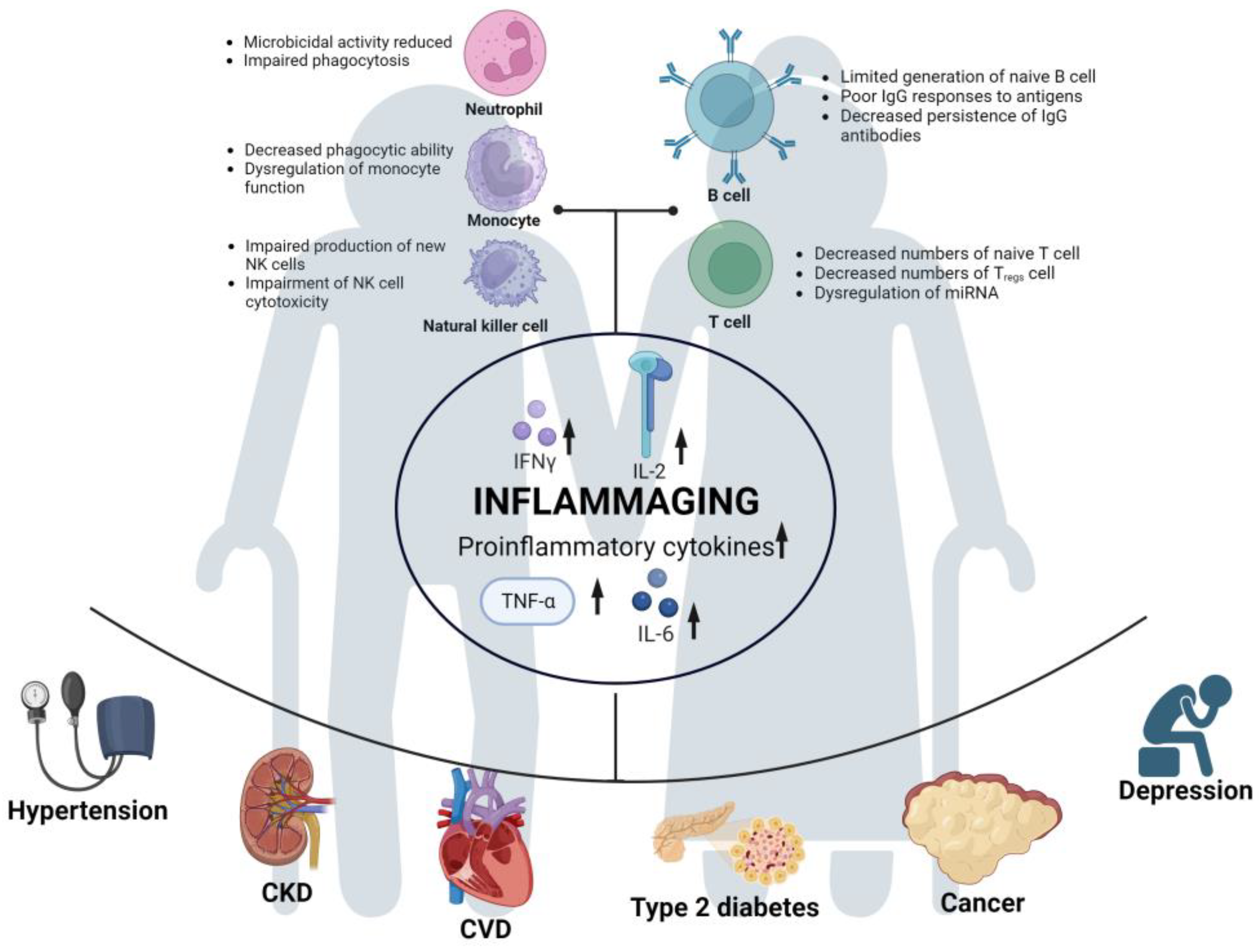
Figure 2. Risk factors for long COVID development in older adults. Immunosenescence in the elderly population leads to immune dysregulation, resulting in reduced or dysfunctional functions of various immune cells. Subsequently, this leads to the upregulation of various proinflammatory cytokines, giving rise to a state of “inflammaging” in the aging immune system. This chronic and long-term inflammatory state makes older adults susceptible to a wide range of comorbidities. It further increases the risk of COVID-19 and long COVID in the elderly population.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11113002
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 26 October 2023).
- Larsen, J.R.; Martin, M.R.; Martin, J.D.; Kuhn, P.; Hicks, J.B. Modeling the Onset of Symptoms of COVID-19. Front. Public Health 2020, 8, 473.
- LitCovid. Available online: https://www.ncbi.nlm.nih.gov/research/coronavirus/ (accessed on 31 May 2023).
- Brugliera, L.; Spina, A.; Castellazzi, P.; Cimino, P.; Tettamanti, A.; Houdayer, E.; Arcuri, P.; Alemanno, F.; Mortini, P.; Iannaccone, S. Rehabilitation of COVID-19 patients. J. Rehabil. Med. 2020, 52, jrm00046.
- WHO. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus; World Health Organization: Geneva, Swizerland, 2021.
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 31 May 2023).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144.
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568.
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314.
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541.
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Author Correction: Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 408.
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205.
- Goldberg, E.M.; Southerland, L.T.; Meltzer, A.C.; Pagenhardt, J.; Hoopes, R.; Camargo, C.A., Jr.; Kline, J.A. Age-related differences in symptoms in older emergency department patients with COVID-19: Prevalence and outcomes in a multicenter cohort. J. Am. Geriatr. Soc. 2022, 70, 1918–1930.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943.
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776.
- Salje, H.; Tran Kiem, C.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hoze, N.; Richet, J.; Dubost, C.L.; et al. Estimating the burden of SARS-CoV-2 in France. Science 2020, 369, 208–211.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059.
- Puhach, O.; Meyer, B.; Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol. 2023, 21, 147–161.
- Chen, X.; Zhu, B.; Hong, W.; Zeng, J.; He, X.; Chen, J.; Zheng, H.; Qiu, S.; Deng, Y.; Chan, J.C.N.; et al. Associations of clinical characteristics and treatment regimens with the duration of viral RNA shedding in patients with COVID-19. Int. J. Infect. Dis. 2020, 98, 252–260.
- Xu, K.; Chen, Y.; Yuan, J.; Yi, P.; Ding, C.; Wu, W.; Li, Y.; Ni, Q.; Zou, R.; Li, X.; et al. Factors Associated With Prolonged Viral RNA Shedding in Patients with Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 799–806.
- Li, T.Z.; Cao, Z.H.; Chen, Y.; Cai, M.T.; Zhang, L.Y.; Xu, H.; Zhang, J.Y.; Ma, C.H.; Liu, Y.; Gao, L.J.; et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J. Med. Virol. 2021, 93, 506–512.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Kim, S.M.; Hwang, Y.J.; Kwak, Y. Prolonged SARS-CoV-2 detection and reversed RT-PCR results in mild or asymptomatic patients. Infect. Dis. 2021, 53, 31–37.
- Zhu, T.; Wang, Y.; Zhou, S.; Zhang, N.; Xia, L. A Comparative Study of Chest Computed Tomography Features in Young and Older Adults With Corona Virus Disease (COVID-19). J. Thorac. Imaging 2020, 35, W97–W101.
- Gu, Q.; Ouyang, X.; Xie, A.; Tan, X.; Liu, J.; Huang, F.; Liu, P. A retrospective study of the initial chest CT imaging findings in 50 COVID-19 patients stratified by gender and age. J. Xray Sci. Technol. 2020, 28, 875–884.
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section CT and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia. Korean J. Radiol. 2020, 21, 746–755.
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up. Radiology 2021, 301, E396–E405.
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33.
- Kim, Y.I.; Yu, K.M.; Koh, J.Y.; Kim, E.H.; Kim, S.M.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.G.; Song, M.S.; et al. Age-dependent pathogenic characteristics of SARS-CoV-2 infection in ferrets. Nat. Commun. 2022, 13, 21.
- Speranza, E.; Purushotham, J.N.; Port, J.R.; Schwarz, B.; Flagg, M.; Williamson, B.N.; Feldmann, F.; Singh, M.; Perez-Perez, L.; Sturdevant, G.L.; et al. Age-related differences in immune dynamics during SARS-CoV-2 infection in rhesus macaques. Life Sci. Alliance 2022, 5, e202101314.
- Blair, R.V.; Vaccari, M.; Doyle-Meyers, L.A.; Roy, C.J.; Russell-Lodrigue, K.; Fahlberg, M.; Monjure, C.J.; Beddingfield, B.; Plante, K.S.; Plante, J.A.; et al. Acute Respiratory Distress in Aged, SARS-CoV-2-Infected African Green Monkeys but Not Rhesus Macaques. Am. J. Pathol. 2021, 191, 274–282.
- Zheng, H.Y.; He, X.Y.; Li, W.; Song, T.Z.; Han, J.B.; Yang, X.; Liu, F.L.; Luo, R.H.; Tian, R.R.; Feng, X.L.; et al. Pro-inflammatory microenvironment and systemic accumulation of CXCR3+ cell exacerbate lung pathology of old rhesus macaques infected with SARS-CoV-2. Signal. Transduct. Target. Ther. 2021, 6, 328.
- Yu, P.; Qi, F.; Xu, Y.; Li, F.; Liu, P.; Liu, J.; Bao, L.; Deng, W.; Gao, H.; Xiang, Z.; et al. Age-related rhesus macaque models of COVID-19. Anim. Model Exp. Med. 2020, 3, 93–97.
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716.
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 2017, 102, 977–988.
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15.
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436.
- Lothar Rink, I.W. Immunosenescence. In Encyclopedia of Infection and Immunity; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 1, pp. 259–276.
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005, 579, 2035–2039.
- Franceschi, C.; Monti, D.; Barbieri, D.; Grassilli, E.; Troiano, L.; Salvioli, S.; Negro, P.; Capri, M.; Guido, M.; Azzi, R.; et al. Immunosenescence in humans: Deterioration or remodelling? Int. Rev. Immunol. 1995, 12, 57–74.
- Barbe-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 2020, 42, 545–557.
- Butcher, S.; Chahal, H.; Savey, E.; Killampalli, V.V.; Alpar, E.K.; Lord, J.M. Functional Decline in Human Neutrophils with Age. Sci. World J. 2001, 1, 67.
- Simell, B.; Vuorela, A.; Ekstrom, N.; Palmu, A.; Reunanen, A.; Meri, S.; Kayhty, H.; Vakevainen, M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011, 29, 1929–1934.
- Dodig, S.; Cepelak, I.; Pavic, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 030501.
- Hearps, A.C.; Martin, G.E.; Angelovich, T.A.; Cheng, W.J.; Maisa, A.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012, 11, 867–875.
- Le Garff-Tavernier, M.; Beziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debre, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535.
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343.
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078.
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194.
- Bulati, M.; Buffa, S.; Candore, G.; Caruso, C.; Dunn-Walters, D.K.; Pellicano, M.; Wu, Y.C.; Colonna Romano, G. B cells and immunosenescence: A focus on IgG+IgD-CD27- (DN) B cells in aged humans. Ageing Res. Rev. 2011, 10, 274–284.
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574.
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015, 194, 4073–4080.
- Stulnig, T.; Maczek, C.; Bock, G.; Majdic, O.; Wick, G. Reference intervals for human peripheral blood lymphocyte subpopulations from ’healthy’ young and aged subjects. Int. Arch. Allergy Immunol. 1995, 108, 205–210.
- Zanni, F.; Vescovini, R.; Biasini, C.; Fagnoni, F.; Zanlari, L.; Telera, A.; Di Pede, P.; Passeri, G.; Pedrazzoni, M.; Passeri, M.; et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: A contribution to understand the relationship between inflammation and immunosenescence. Exp. Gerontol. 2003, 38, 981–987.
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137.
- Kroesen, B.J.; Teteloshvili, N.; Smigielska-Czepiel, K.; Brouwer, E.; Boots, A.M.; van den Berg, A.; Kluiver, J. Immuno-miRs: Critical regulators of T-cell development, function and ageing. Immunology 2015, 144, 1–10.
- Franceschi, C. Inflammaging as a major characteristic of old people: Can it be prevented or cured? Nutr. Rev. 2007, 65, S173–S176.
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827.
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567.
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522.
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9.
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185.
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate immune receptors: Key regulators of metabolic disease progression. Cell Metab. 2013, 17, 873–882.
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184.
- Hansen, P.R. Chronic Inflammatory Diseases and Atherosclerotic Cardiovascular Disease: Innocent Bystanders or Partners in Crime? Curr. Pharm. Des. 2018, 24, 281–290.
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380.
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899.
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905.
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742.
- Lamers, F.; Milaneschi, Y.; de Jonge, P.; Giltay, E.J.; Penninx, B. Metabolic and inflammatory markers: Associations with individual depressive symptoms. Psychol. Med. 2018, 48, 1102–1110.
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55.
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538.
- Cai, Q.; Huang, D.; Ou, P.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Ma, Z.; Zhang, Y.; Li, Z.; et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020, 75, 1742–1752.
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020, 92, 797–806.
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337.
- Wang, L.; Li, X.; Chen, H.; Yan, S.; Li, D.; Li, Y.; Gong, Z. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am. J. Nephrol. 2020, 51, 343–348.
- Djaharuddin, I.; Munawwarah, S.; Nurulita, A.; Ilyas, M.; Tabri, N.A.; Lihawa, N. Comorbidities and mortality in COVID-19 patients. Gac. Sanit. 2021, 35 (Suppl. S2), S530–S532.
- Bae, S.; Kim, S.R.; Kim, M.N.; Shim, W.J.; Park, S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021, 107, 373–380.
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 2023, 56, 1–9.
- Zhang, X.; Wang, F.; Shen, Y.; Zhang, X.; Cen, Y.; Wang, B.; Zhao, S.; Zhou, Y.; Hu, B.; Wang, M.; et al. Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge From Hospitals in Wuhan, China. JAMA Netw. Open 2021, 4, e2127403.
This entry is offline, you can click here to edit this entry!
