Dual centrifugation (DC) is an innovative in-vial homogenization and in-vial nanomilling technique that has been in use for the preparation of liposomes. DC has continuously been developed for preparing various liposomes and other lipid nanoparticles including emulsions and solid lipid nanoparticles (SLNs) as well as polymersomes and nanocrystals. Improvements in equipment technology have been achieved, so that DC is now on its way to becoming the quasi-standard for the simple, fast, and aseptic production of lipid nanoparticles and nanocrystals in small and medium batch sizes, including the possibility of simple and fast formulation screening or bedside preparations of therapeutic nanoparticles.
1. Introduction
DC is a unique process in which a sample vial in a fast-running centrifuge (primary rotation) is additionally turned around a second axis (secondary rotation)
[1]. As a result, the direction of the high centrifugal acceleration continuously changes in relation to the (turning) sample vial, which results in highly frequent and, at the same time, strong movements of the sample material inside the vial, which typically contains heavy ZrO-beads to support the process
[2]. The very intense sample movements can principally be used for mixing, shaking, milling, or homogenizing. This research focuses on the preparation of lipid and polymer nanoparticles such as liposomes, emulsions, solid lipid nanoparticles, or polymersomes using DC as a tool for in-vial homogenization and of nanocrystals using DC as a tool for in-vial nanomilling.
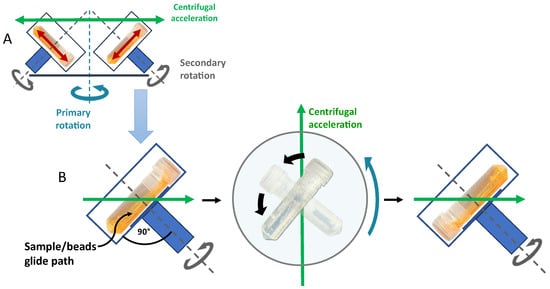
In addition to the high centrifugal acceleration of the samples due to a fast primary rotation and an optimal turning frequency of the sample vial around the second rotational axis, the use of lengthy vials that are placed into the dual rotor at a 90° angle to the axis of the second rotation (horizontal vial positioning, compare Figure 1) is ideal for introducing the maximal energy into the sample material. Due to the very high in-vial homogenization and milling performance, this research is restricted to DC using lengthy vials and the horizontal vial positioning, which, however, includes virtually all previous publications on lipid and polymer nanoparticles prepared with DC.
Figure 1. Schematic representation of the basic principles of dual centrifugation using horizontal vial positioning (90° to the second rotation axis). (A) Dual rotor with primary and secondary rotation. (B) Sample movement in a dual centrifuge due to constant centrifugal acceleration in combination with sample vial rotation. Green arrows show the direction of the constantly effective centrifugal acceleration.
One important aspect explaining the impressive homogenization or milling results is the fact that the horizontal vial orientation gives the sample inside the vial the maximal way to accelerate (
Figure 1 and
Figure 2), resulting in the strongest impact when the sample containing heavy ZrO-beads reaches the end of the vial. This sample movement is in a certain way comparable to that of a horizontal laboratory shaker, with the important difference that the sample acceleration during DC is more than two orders of magnitude higher and therefore strong enough for efficient homogenization or nanomilling
[2].
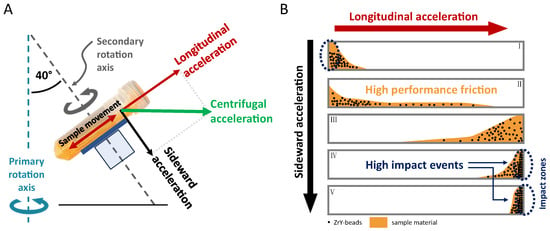
Figure 2. Homogenization principles during DC: (A) Centrifugal acceleration during DC is split into a vector that moves the sample along the sample vial (longitudinal acceleration), and a vector that pushes the sample to one side of the inner wall of the sample vial (sideward acceleration). (B) Longitudinal acceleration causes sample movement resulting in a strong impact when the mixture of sample and beads reaches the end of the vial (impact zone, right part of the figure, Ⅳ and Ⅴ). In addition to that, the unique interaction of longitudinal and sideward acceleration forces the mixture of sample and beads to a defined glide path while moving from top to bottom of the lengthy vial, which additionally causes high-performance friction (sample glide path, distribution of the sample material is shown in orange color: B, mainly Ⅱ and Ⅲ).
The second important aspect explaining the good homogenization efficacy using lengthy vials in combination with a horizontal vial positioning is that the sample does not “fly” directly from top to bottom. Instead, the sample including the ZrO-beads glides along one side of the inner vial wall from top to bottom and vice versa. Since the axes of the secondary rotation have an approx. 40° angle related to the main (primary) rotational axis (compare Figure 2), the sample gliding takes place on a defined path inside the vials. The explanation for this is that the vector of centrifugal acceleration hits the vessel at this 40° angle and thus acts on the sample in two directions. One vector of the parallelogram of forces pushes the sample from top to bottom of the vial, and the other vector presses the sample on the gliding path (Figure 2). This results in additional “high-performance friction” of the sample by the heavy ZrO-beads.
2. Dual Centrifuges
Dual centrifuges that have successfully been used for the preparation of nanoparticles using horizontally orientated vials are the SpeedMixer® DAC 150 (DAC 150, Hauschild GmbH & Co. KG, Hamm, Germany), the ZentriMix 380 R (ZM 380R, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany), and the DeltaVita 1 (DV1, Erich Netzsch GmbH & Co. Holding KG, Selb, Germany; identical in construction to ZentriMix 380 R).
The SpeedMixer
® DAC 150 (DAC 150) was initially designed for very short runs of a few seconds to minutes to mix highly viscous compounds. Thus, this device is equipped with a strong motor that requires a V-belt to couple the main and secondary rotation. Using a V-belt protects the mechanics from slippage when the dual asymmetric centrifuge starts. Despite its maximum run time being 5 min, the DAC 150 can be used to prepare nanoparticles
[1] if the necessary run times (typically about 30 min) are reached by multiple short runs. However, standard vial adapters are only available for vertical vial orientation, which is optimal for fast mixing of highly viscous materials such as print inks, silicones, or two-component teeth filling materials
[2]. Adapters for the horizontal orientation of small and lengthy vials are not commercially available for the DAC 150 and are all custom made for patent reasons. Since the diameter of the rotational disk to accommodate the sample vial adapter is rather small, only a few vials (typically two) can be processed simultaneously.
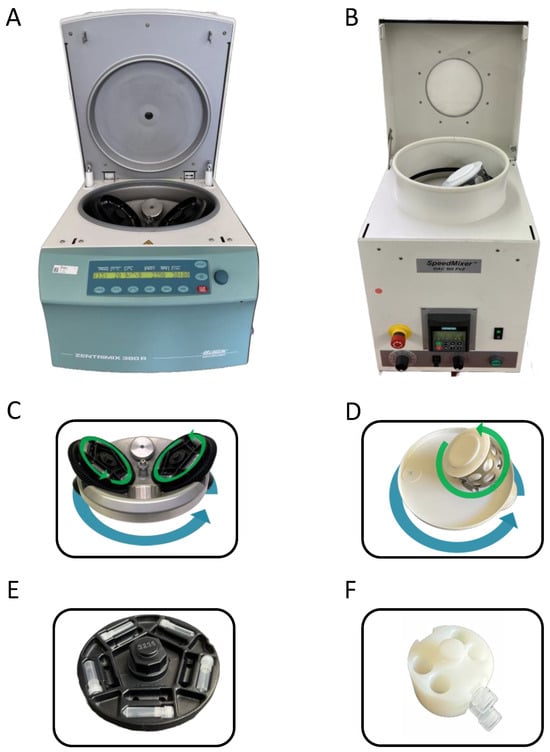
A special feature of the DAC 150 is that its rotor is asymmetrically constructed with only one sample holder (Figure 3). Thus, smooth running of this rotor is ensured by a fixed counterweight; as a result, the payload is also fixed to a certain weight. If very small sample quantities are processed, the necessary weight of the payload must be reached by a special (heavy) sample holder. Based on using the DAC 150 with its special asymmetric rotor for the preparation of nanoparticles, in a few publications, the DC-process is named “dual asymmetric centrifugation (DAC)”. In contrast to that, the other dual centrifuges (DV1 and ZM 380R) have symmetric dual rotors with two symmetric sample holders. Therefore, the term “dual centrifugation (DC)” refers to the fact that there are two types of rotational axes within one rotor—one axis for the main rotation of the whole DC-rotor, and a second set of axes for turning the sample vials (compare Figure 3).
Figure 3. Comparison of DC-devices used for the preparation of nanosized pharmaceutical formulations using the horizontal vial positioning. (A) ZentriMix 380 R from Hettich with the corresponding DC-rotor (C) and adapter for horizontal positioning of ten 2 mL vials on one level (in total 40 vials in one run possible) (E). The ZentriMix-rotor is removeable and can be replaced by a normal centrifugal rotor for using the device as normal centrifuge. The DV1 device from Netzsch is identical in construction. (B) DAC 150 from Hauschild with corresponding DAC-rotor (D) and customized adapter for horizontal orientation of 2 mL vials (F).
The dual centrifuges DV1 and ZM 380R are designed from the outset to produce nanoparticles, which typically requires longer run times than a few minutes. Thus, the DC-processing time is freely adjustable. Furthermore, the coupling of the main and secondary rotations is established by gear wheels, and the motor control allows a soft start to protect the mechanics. To dissipate the heat inevitably generated during DC-homogenization or -nanomilling, DV1 and ZM 380R are equipped with a powerful cooling unit. Furthermore, dual rotors of DV1 and ZM 380R have rather large rotational disks to accommodate a high number of the typically used 2 mL PP-screw cap vials (maximum 40 vial per run, arranged on 2 levels with 20 vials each), which is advantageous for formulation screening approaches, for example. Due to the frequent change in the direction of the high centrifugal acceleration during DC, all types of vials require special adapters to adequately fix the vials in place (Figure 3E,F).
However, with otherwise equally fast secondary rotation (about 850 rpm at max. speed of main rotation), the centrifugal acceleration of the ZM 380R is about 15% higher compared to the DAC 150, which is due to the larger rotor diameter that allow faster acceleration of the sample materials. Sample materials with higher viscosities thus have a better chance of reaching the end of the vials before the vial has turned again in the opposite direction. Since a significant part of the homogenization or milling processes take place at the end of the sample vials due to collisions (compare Figure 2, impact zones), reaching the end of the vials results in more effective homogenization or milling processes.
3. Beads in Use for Dual Centrifugation
Homogenization or nanomilling is typically supported by spherical ceramic beads with a high density (ZrO-beads, above 6.0 kg/dm
3) since heavy beads accumulate very high kinetic energies during acceleration and thus introduce more energy into the sample material. In the first studies, liposome preparation was performed by DC-homogenization using much lighter glass beads (approx. 2.23 kg/dm
3 for borosilicate glass), which were used with satisfactory results on first glance
[1]. However, it turned out that the numerous collisions between the glass beads resulted in glass wear, which, due to the basic surface properties of the freshly generated glass (nano)particles (containing potassium-oxide), caused degradation of the phosphatidylcholine head groups. The proposed underlying chemical reaction might be the well-known Hoffmann elimination, which started with the removal of a proton in β-position to the quaternary amino group of phosphatidylcholine-species by the basic glass (nano)particles, followed by a rearrangement of bonds. The final reaction product is trimethylamine, which can easily be detected by its fishy smell, even in small quantities
[2]. Thus, the use of the much denser and harder ceramic beads was established and almost all studies cited in this research used those beads.
Commonly used ceramic beads are made from zirconium-oxide, which are stabilized with yttrium. An example are SiLibeads type ZY-P Pharma purchased from Sigmund Lindner GmbH (Warmensteinach Germany). Their high degree of roundness (≥0.96 (width to length ratio (x
min/x
max)) and polished surface avoids the generation of wear from the commonly used plastic vials (see below). The high hardness (microhardness: ≥1300 HV10) contributes to the avoidance of zirconia wear during bead–bead collisions as well. In a DC-nanomilling study, a zirconium amount of only 3.4 ppm was found in the resulting suspension after 90 min milling time, which was shown to be comparable with wear generated from ZrO-beads during agitator ball milling in pharmaceutical production
[3].
For DC-homogenization, typical bead diameters range from 0.2 to 1.6 mm. However, the optimal bead size and number (bigger beads) or amounts (smaller beads) vary depending on the specific lipid blend or nanocrystal dispersion and the vial type. Smaller and thus lighter beads introduce less energy during bead–bead collisions but increase the number of bead–bead collisions. Therefore, the bead size and quantity must be optimized for each DC-process.
4. Vials in Use for Dual Centrifugation
One important aspect of successful dual centrifugation is that the homogenization/milling vials have to be made of a slightly elastic plastic material. In most cases, disposable 2 mL screw cap polypropylene (PP) vials are in use in combination with ZrO-beads. Another important aspect of choosing the right vials for DC concerns their tightness and stability. The vials must be very tightly sealed and stable enough since during DC, the sample material and the beads are pushed with high impact and at high frequency against the lid and the bottom (compare
Figure 2). Therefore, it is important to either use the exact vials described in the existing publications or carefully check the tightness of new vials that have not yet been used in test runs. As an alternative, 10 mL PP-injection vials at the same length as the screw cap vials can be used
[2].
Considering wears from the surface of the plastic (PP) vials has not been observed or reported so far when using 2 mL screw cap vials in combination with ZrO-beads of high quality (see above). This could be explained by the high degree of roundness in combination with the smooth, polished surface of the ZrO-beads, which, even when in contact with the plastic walls of the vials, are assumed to not be able to effectively scratch the plastic material. In the case of liposome preparation by DC, the danger of scraping of the plastic walls is further reduced by the lubrication effect of the highly viscous and concentrated lipid blends. Thus far, systematic studies focusing on the generation of wear (PP or ZrO) during the preparation of liposomes within a DC do not exist.
This entry is adapted from the peer-reviewed paper 10.3390/ph16111519