Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Living organisms rely on pH levels for a multitude of crucial biological processes, such as the digestion of food and the facilitation of enzymatic reactions. Among these organisms, animals, including insects, possess specialized taste organs that enable them to discern between acidic and alkaline substances present in their food sources.
- taste
- acid
- alkali
- OTOP1
- Alkaliphile
1. Introduction
Sensory perception plays a vital role in the survival and well-being of animals, enabling them to navigate their environment and meet their fundamental requirements, such as securing nourishment, finding shelter, ensuring reproductive success, ensuring safety, and engaging in meaningful interactions with other members of their ecosystem. This complex procedure requires the synchronization of multiple separate sensory systems, each skilled in transmitting vital data to the brain for prompt analysis and reaction. Among these, mammals have evolved five primary sensory organs, namely the eye for vision, the ear for hearing, the tongue for taste, the nose for smell, and the skin for tactile perception, collectively facilitating the discernment of visual cues, auditory signals, flavors, scents, and tactile sensations. Much like other mammals, Drosophila melanogaster possesses distinct sensory organs that empower it to distinguish favorable from unfavorable environmental stimuli, enabling its survival. Drosophila exhibits a sophisticated sensory apparatus that aids in the recognition of external stimuli. Their remarkable compound eyes are particularly adept at facilitating various aspects of visual behavior, serving as a pivotal tool for environmental navigation and identification [1]. Furthermore, the species relies on the Johnston’s organ, situated within the antenna, which serves the dual purpose of detecting sounds and facilitating mechanosensation, thereby allowing fruit flies to respond to auditory and mechanical stimuli with precision [2][3][4][5]. In addition to these, Drosophila possesses a diverse array of olfactory organs, each specialized in the detection and processing of a wide range of odors [6]. These olfactory receptors play a critical role in the fly’s ability to identify and respond to specific volatile chemical cues within their surroundings. Moreover, the gustatory organs of the fruit fly enable the perception of taste, allowing them to differentiate between various food sources and potentially harmful substances [7][8][9][10][11][12]. This intricate sensory system collectively equips Drosophila with the tools necessary for efficient perception and response to the external environment. Within the adult body of Drosophila, hair-like projections develop into taste organs in various locations, including the proboscis, legs, wing margins, and ovipositor [13][14][15][16][17][18][19]. Of these, the tip of the proboscis comprises the bifurcate labellum, which plays a particularly crucial role in detecting taste by coming into contact with food or chemical compounds. The labellum of Drosophila contains 31 taste sensilla on each side, arranged symmetrically. These sensilla are instrumental in chemosensations, especially concerning non-volatile compounds. There are three distinct types of sensilla in the labellum, short (S-type), intermediate (I-type), and long (L-type), differentiated by their size [16][20][21]. Each sensillum is innervated by two to four gustatory receptor neurons (GRNs), one mechanosensory neuron, and three supporting cells, with their signals projected to the subesophageal zone (SEZ) of the brain, the region responsible for taste sensation [13][22][23][24]. Additionally, taste sensation is also mediated by the hairless labellar taste peg situated between pseudotracheal rows, which is innervated by one chemosensory neuron and one mechanosensory neuron [25]. It is believed that taste pegs can only detect food when the flies open their labial palps. Furthermore, the adult fly pharynx, which acts as an internal molecular sensor, contains three different hairless internal taste organs: the dorsal cibarial sense organ (DCSO), the ventral cibarial sense organ (VCSO), and the labral sense organ (LSO) [26].
More precisely, the presence of specific molecular components like gustatory receptors (GRs) [7][27], ionotropic receptors (IRs) [28][29], pickpocket (PPK) ion channels [30][31][32], and transient receptor potential (TRP) ion channels [33][34] acts as mediators between external chemical cues and the fruit fly’s brain. These components play a crucial role in transducing chemical information into neural signals. The GRNs present in sensilla are sensitive to different types of chemical stimuli entering via pores at the tip of the sensilla. For example, the S-type sensilla contains four different GRNs, each responding to bitter or aversive compounds, sweet tastes, water, or specific minerals such as Na+ and Ca2+ [13][18][19][32]. In contrast, the L-type sensilla possesses four sets of GRNs that are sensitive to sweet tastes, water, low concentrations of salt, and others which have not yet been identified [19][35][36][37][38]. Similarly, the I-type sensilla are equipped with two sets of GRNs responding to sweet and bitter compounds, including both low and high concentrations of salt [39][40][41]. In this intricate web of molecular and sensory interactions, Drosophila’s taste organs play a pivotal role in helping the fly navigate its environment, ensuring its sustenance and survival. These sensory mechanisms provide a fascinating window into the broader world of biological systems and their adaptation to complex ecological challenges.
Taste preferences in Drosophila also have profound ecological implications. They guide foraging behavior, influence food source selection, impact breeding site choices, and even contribute to competition and niche partitioning among different Drosophila populations. Drosophila’s taste neurons and receptors assess food sources, shaping their preferences and guiding foraging. Favorable food sources lead to concentrated fly populations, impacting resource distribution. Drosophila can also act as pollinators, spreading pollen from one plant to another as they feed on nectar, affecting plant ecology. Taste cues guide females to identify and select appropriate substrates for oviposition. Consequently, taste preferences influence the distribution of Drosophila larvae within their environment, impacting their development and survival. Diverse taste perception facilitates niche partitioning, enabling the coexistence of multiple Drosophila species by exploiting different food sources and reducing competition. Understanding these taste preferences is essential for unraveling the intricate ecological dynamics and interactions between these fruit flies and their environment.
Drosophila’s food preferences go beyond taste alone, influenced by nutritional needs, genetic variation, environmental conditions, and learned behavior. While taste guides their initial preferences, nutritional requirements can override taste, and genetic diversity affects their perception of specific compounds [42][43]. Environmental factors, like food availability and competition, also play a significant role. Drosophila can learn from experience, allowing them to adapt and make optimal food choices. These multifaceted factors ensure their adaptability and reproductive success in diverse environments.
Sourness, characterized by low pH or acidity, represents a fundamental taste sensation [44]. It is universally appealing in moderate concentrations, yet becomes unappealing at higher levels, across both vertebrate and invertebrate species [45][46][47][48]. Conversely, foods with high pH or alkaline properties generally lack appeal [49][50][51]. Nevertheless, in mildly alkaline conditions, food with compounds like ammonia and certain amines is preferred, influenced by the intricacies of the sensory system [52][53] (Figure 1A). Notably, the attraction to mildly basic food is believed to be linked to the presence of low concentrations of salt [49]. Commonly, acidic components are found in raw fruits and spoiled food items, adding to the significance of detecting sourness as a warning sign. Conversely, basic or alkaline taste is triggered in certain vegetables, legumes, nuts, and other items due to their elevated pH levels. Current research across various species, both vertebrate and invertebrate, has substantiated that basic taste also qualifies as one of the fundamental taste qualities [49][51][54][55][56].
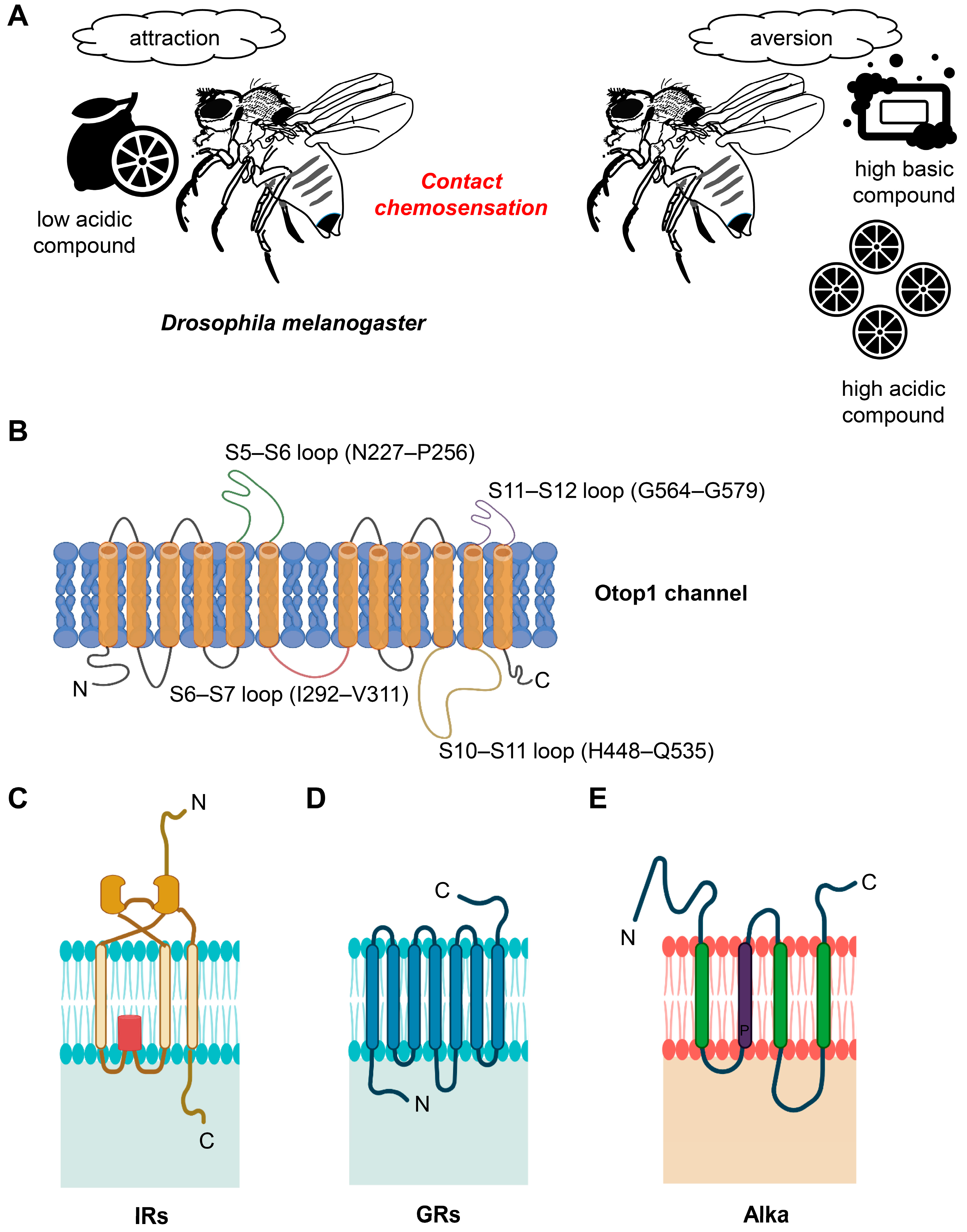
Figure 1. Behavior of acid and basic compounds and structure of OTOP1, GRs, IRs, and Alka. (A) Representation of D. melanogaster’s sensitivity to pH Levels. D. melanogaster is attracted to compounds with low acidity (low pH) while displaying aversion to highly acidic and highly basic compounds (high pH). (B) Human Otop1 channel membrane configuration. The human Otop1 channel is thought to have a membrane-spanning structure, with the N domain encompassing transmembrane segments S1 through S6, the C domain including S7 to S12, interconnected loops between these segments, and intracellular termini. (C) Architectural features of GRs. GRs possess a unique architectural composition characterized by seven transmembrane domains (TMDs), with their C-terminal regions located externally, distinguishing them from typical G-protein coupled receptors. (D) Topology of IRs. IRs exhibit a three-TMD structure, including a pore region, which shares structural similarities with mammalian glutamate receptors. (E) Proposed structural arrangement of alka. Alka is anticipated to have a structural configuration that includes a persistent proline residue (P) within the TM2 segment.
2. Molecular Mechanism of Acid Sensation
The perception of sour taste, resulting from the presence of acidic compounds in food, has long intrigued scientists, leading to a gradual understanding of how the peripheral taste system in animals detects this taste, alongside other fundamental tastes. Due to variations in the chemical sensitivity of taste receptors among different animal species, identifying a single universal channel responsible for sour taste detection proved challenging [57].
A different study proposed a widely accepted theory that sour taste perception involves acid molecules breaching cell membranes to release hydrogen ions [58][59], activating sour taste receptors in specialized taste receptor cells (TRCs) on the tongue [60][61]. Throughout evolution, animals have developed a diverse array of proton-activated channels and G-protein-coupled receptors (GPCRs) to distinguish acidic pH levels. Various potential receptors were considered, including the acid-sensing ion channel 2 (ASIC2) [62] and hyperpolarization-activated cyclic nucleotide gated (HCN 1 and HCN 4) channels [63][64], but their roles were inconclusive [65][66]. Polycystic kidney disease 2-like 1 (PKD2L1) was initially thought to be a sour taste receptor [67][68], but further studies found its role to be modest [69][70]. Ultimately, Otopetrin 1 (OTOP1) emerged as a pivotal sensor in sour taste perception, marking a significant milestone in the understanding of animal taste sensation [71][72][73] (Figure 1B). Further, researchers discovered that lowering extracellular pH increases inward current in Type III taste receptor cells, indicating their role in detecting sourness [74]. OTOP1 is identified as essential in sour taste transduction, with experiments confirming pH-dependent currents [71][74]. Cryo-EM analysis revealed OTOP1’s dimeric structure with 12 transmembrane helices [75]. The mechanism of OTOP1 gating for proton permeation remains unknown, warranting future research. Fruit flies possess Otopetrin-like a (Otop-La) as a functional ortholog of OTOP1 [46][47]. Otopetrins are a family of proteins that have been implicated in sour taste perception in various organisms, including mammals and fruit flies [46][47]. This protein is part of the molecular machinery that allows fruit flies to perceive and respond to sour tastes, ensuring their ability to make informed dietary choices. Understanding the presence of Otop-La in fruit flies adds to the knowledge of the molecular mechanisms underlying sour taste perception in insects, contributing to the broader understanding of taste sensation across different species.
Behavioral analysis in fruit flies demonstrated a bidirectional response to diets with varying acid concentrations, showing attraction to low-acid and aversion to highly acidic food [45][48][76]. Electrophysiological analyses revealed that L-type sensilla were responsible for the attraction to acid-containing foods, while S-type sensilla were involved in exhibiting aversion [45][48][76]. Nevertheless, this outcome does not imply that L-type sensilla solely responds to attractive compounds such as low pH compound, while S-type sensilla is solely responsible for exhibiting neuronal response to aversive compounds like high pH compound. Researchers showed that IR7c-expressing GRNs, which are required to sense high concentrations of salt (aversive nature), are expressed in most of the L-type sensilla and few S-type sensilla [37]. This suggests that even L-type sensilla could serve as a neuronal responder, contributing to the detection of a compound known to induce aversive behavior. In addition, sensilla S4 and S8, akin to L-type sensilla, demonstrated heightened neuronal responses to appealing concentrations of salt, underscoring the likelihood that S-type sensilla also exhibited increased neuronal activity in response to enticing compounds [77]. Thus, different sensilla exhibit different neuronal response for various tastants, including acidic compounds, and the exact connectome between S-type and L-type sensilla is yet to be established.
3. Alkali Detection in Taste
Just as low pH (acidity) produces a gustatory sensation known as sourness, it is logical to hypothesize that high pH (alkalinity) might also elicit a distinct taste sensation. Early scientific inquiries suggested that alkali compounds could evoke specific types of tastes, such as sour and sweet, by exciting taste receptors [78][79]. For example, studies dating back to 1948 observed that the tip of the human tongue had a higher sensitivity to sodium hydroxide solution than other regions, providing early evidence for an alkaline taste sensation [54]. Electrophysiological recordings of taste nerves in cats also indicated that a subset of these nerves could be activated by high pH, suggesting the existence of an alkaline taste [55]. Trigeminal neurons, a type of sensory neurons, respond to various external alkaline pH levels [55]. Among the TRP channels, TRPV1 and TRPA1 are known to be activated by intracellular alkalization but not by exposure to external alkaline pH alone [80][81]. It is important to note that these channels can also be activated by acidic pH levels.
In the context of studying C. elegans, TMC-1 plays a role in mediating alkaline sensation through ASH nociceptive neurons [82]. TMC-1 is one of the two TMC family genes found in C. elegans and is believed to encode a sodium-sensitive channel that is required for salt chemosensation and food signaling. When exposed to alkaline pH, ASH neurons exhibit an inward current that is primarily dependent on TMC-1 and only secondarily dependent on the TRPV channel OSM-9. While OSM-9/TRPV is sensitive to both acidic and basic pH, TMC-1 displays specificity towards alkaline conditions. It is essential not only for the electrical current in ASH neurons, but also for the behavioral response triggered by alkaline pH, whereas it is not involved in the response to acidic pH [82].
Research on carabid beetles and ground beetles demonstrated that these insects exhibit a strong aversion to alkaline conditions, particularly in relation to their habitats and food sources [83]. These studies suggested that insects have taste receptors that can detect high pH levels and influence their feeding behavior. Furthermore, this study holds ecological significance, as the aversion to alkaline conditions influences habitat selection, affects soil quality and vegetation composition, impacts community dynamics, and holds implications for conservation and land management practices across diverse ecosystems. It highlights the intricate web of interactions and dependencies that characterize ecological systems. In the context of Drosophila research, it was discovered that alkaline substances with a high pH could indeed generate a gustatory sensation, implying the presence of a separate channel in the taste organ dedicated to alkaline taste perception. Further investigation revealed the importance of a chloride channel called Alkaliphile (Alka) in fruit flies’ adverse taste reactions to basic foods (Figure 1E). Alka selectively creates a high pH-gated chloride channel in specific GRNs, enabling the detection of alkaline taste [49]. Additionally, it was found that high pH conditions suppressed the sugar-based neuronal response triggered by sweet-sensing GRNs, supporting the notion that the detection of high pH involves dual mechanisms: the activation of certain bitter-sensing GRNs and the inhibition of sweet-sensing GRNs. This discovery in fruit flies has paved the way for future studies exploring how other organisms’ peripheral taste organs perceive alkaline tastes at the molecular level.
Additionally, research in vertebrates, specifically zebrafish, revealed the involvement of OTOP1 in basic taste sensation [51]. OTOP1 was found to mediate proton inflow and efflux in response to extracellular acid and base stimulation. Notably, the mutation of specific domains within OTOP1 affected its affinity for alkali compounds without impacting its response to acidic stimuli, highlighting the distinction between acid and alkali activation [51]. The mouse study demonstrated that OTOP1 functions as a sensor for ammonium chloride (NH4Cl) [84]. Taste responses to NH4Cl, as measured from isolated Type III TRCs or gustatory nerves, were significantly reduced or completely absent in an Otop1−/− mouse. Recent research into the gustatory system using Drosophila and Aedes aegypti has found that the perception of sweetness and saltiness can be inhibited as the basicity (pH) of a tastant solution increases, particularly with the addition of ammonium hydroxide (NH4OH) [50]. This intriguing discovery challenges the understanding of taste, and holds potential implications for industries like food and beverage industries, as it suggests that altering basicity could influence flavor perception and innovation.
In the context of acidic food conditions, an intriguing research avenue emerges with adaptability based on the changing condition of internal body state [85]. Exploring the impact of alkaline presence on internal states and consequent behavioral changes presents an exciting opportunity for investigation. Delving deeper, it becomes fascinating to examine how these state-dependent alterations influence the functioning of sensory neurons, particularly in the sugar and bitter circuits downstream. Additionally, a compelling aspect of this research could involve studying the switch in response to hunger levels. This may explain how organisms adapt to alkaline food, which could be considered a potentially aversive compound, yet may offer nutritional benefits in extreme conditions. The exploration of alkaline taste, like sour and other taste modalities, continues to unravel the intricacies of the gustatory system and how organisms perceive and respond to a wide range of chemical stimuli in their environment.
This entry is adapted from the peer-reviewed paper 10.3390/metabo13111131
References
- Bausenwein, Á.; Dittrich, A.; Fischbach, K.-F. The optic lobe of Drosophila melanogaster: II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 1992, 267, 17–28.
- Tuthill, J.C.; Wilson, R.I. Mechanosensation and adaptive motor control in insects. Curr. Biol. 2016, 26, R1022–R1038.
- Nadrowski, B.; Effertz, T.; Senthilan, P.R.; Göpfert, M.C. Antennal hearing in insects–new findings, new questions. Hear. Res. 2011, 273, 7–13.
- Boekhoff-Falk, G.; Eberl, D.F. The Drosophila auditory system. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 179–191.
- Keil, T.A. Functional morphology of insect mechanoreceptors. MRT 1997, 39, 506–531.
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999, 96, 725–736.
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834.
- Scott, K.; Brady, R.; Cravchik, A.; Morozov, P.; Rzhetsky, A.; Zuker, C.; Axel, R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 2001, 104, 661–673.
- Chyb, S.; Dahanukar, A.; Wickens, A.; Carlson, J.R. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. USA 2003, 100, 14526–14530.
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998.
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244.
- Wang, Z.; Singhvi, A.; Kong, P.; Scott, K. Taste representations in the Drosophila brain. Cell 2004, 117, 981–991.
- Falk, R.; Bleiser-Avivi, N.; Atidia, J. Labellar taste organs of Drosophila melanogaster. J. Morphol. 1976, 150, 327–341.
- Ghysen, A.; Dambly-Chaudiere, C. Genesis of the Drosophila peripheral nervous system. TiG 1989, 5, 251–255.
- Dambly-Chaudière, C.; Jamet, E.; Burri, M.; Bopp, D.; Basler, K.; Hafen, E.; Dumont, N.; Spielmann, P.; Ghysen, A.; Noll, M. The paired box gene pox neuro: A determinant of poly-innervated sense organs in Drosophila. Cell 1992, 69, 159–172.
- Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: A rewiew. Cell Tissue Res. 1994, 275, 3–26.
- Singh, R.N. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. MRT 1997, 39, 547–563.
- Dethier, V.G. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding; Harvard University Press: Cambridge, MA, USA, 1976.
- Shrestha, B.; Lee, Y. Molecular sensors in the taste system of Drosophila. Genes Genom. 2023, 45, 693–707.
- Shanbhag, S.; Park, S.-K.; Pikielny, C.; Steinbrecht, R.A. Gustatory organs of Drosophila melanogaster: Fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 2001, 304, 423–437.
- Hiroi, M.; Marion-Poll, F.; Tanimura, T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zool. Sci. 2002, 19, 1009–1018.
- Power, M.E. The thoracico-abdominal nervous system of an adult insect, Drosophila melanogaster. J. Com. Neurol. 1948, 88, 347–409.
- Stocker, R.; Schorderet, M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981, 216, 513–523.
- Rajashekhar, K.; Singh, R.N. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J. Comp. Neurol. 1994, 349, 633–645.
- Chen, Y.-C.D.; Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. CMLS 2020, 77, 1087–1101.
- Chen, Y.-C.D.; Dahanukar, A. Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep. 2017, 21, 2978–2991.
- Weiss, L.A.; Dahanukar, A.; Kwon, J.Y.; Banerjee, D.; Carlson, J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron 2011, 69, 258–272.
- Zhang, Y.V.; Ni, J.; Montell, C. The molecular basis for attractive salt-taste coding in Drosophila. Science 2013, 340, 1334–1338.
- Rimal, S.; Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Ins. Mol. Biol. 2018, 27, 1–7.
- Cameron, P.; Hiroi, M.; Ngai, J.; Scott, K. The molecular basis for water taste in Drosophila. Nature 2010, 465, 91–95.
- Chen, Z.; Wang, Q.; Wang, Z. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for Drosophila gustatory water reception. J. Neurosci. 2010, 30, 6247–6252.
- Lee, Y.; Poudel, S.; Kim, Y.; Thakur, D.; Montell, C. Calcium taste avoidance in Drosophila. Neuron 2018, 97, 67–74.e64.
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600.
- Kim, S.H.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Montell, C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 8440–8445.
- Fujishiro, N.; Kijima, H.; Morita, H. Impulse frequency and action potential amplitude in labellar chemosensory neurones of Drosophila melanogaster. J. Insect Physiol. 1984, 30, 317–325.
- Nayak, S.V.; Singh, R.N. Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int. J. Insect Morphol. Embryol. 1983, 12, 273–291.
- McDowell, S.A.; Stanley, M.; Gordon, M.D. A molecular mechanism for high salt taste in Drosophila. Curr. Biol. 2022, 32, 3070–3081.e3075.
- Jaeger, A.H.; Stanley, M.; Weiss, Z.F.; Musso, P.-Y.; Chan, R.C.; Zhang, H.; Feldman-Kiss, D.; Gordon, M.D. A complex peripheral code for salt taste in Drosophila. Elife 2018, 7, e37167.
- Rodrigues, V.; Siddiqi, O. Genetic analysis of chemosensory pathway. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1978, 87, 147–160.
- Montell, C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009, 19, 345–353.
- Hiroi, M.; Meunier, N.; Marion-Poll, F.; Tanimura, T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J. Neurobiol. 2004, 61, 333–342.
- Dhakal, S.; Ren, Q.; Liu, J.; Akitake, B.; Tekin, I.; Montell, C.; Lee, Y. Drosophila TRPγ is required in neuroendocrine cells for post-ingestive food selection. Elife 2022, 11, e56726.
- Dus, M.; Min, S.; Keene, A.C.; Lee, G.Y.; Suh, G.S. Taste-independent detection of the caloric content of sugar in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 11644–11649.
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000.
- Rimal, S.; Sang, J.; Poudel, S.; Thakur, D.; Montell, C.; Lee, Y. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 2019, 26, 1432–1442.e1434.
- Mi, T.; Mack, J.O.; Lee, C.M.; Zhang, Y.V. Molecular and cellular basis of acid taste sensation in Drosophila. Nat. Commun. 2021, 12, 3730.
- Ganguly, A.; Chandel, A.; Turner, H.; Wang, S.; Liman, E.R.; Montell, C. Requirement for an Otopetrin-like protein for acid taste in Drosophila. Proc. Natl. Acad. Sci. USA 2021, 118, e2110641118.
- Frank, H.E.; Amato, K.; Trautwein, M.; Maia, P.; Liman, E.R.; Nichols, L.M.; Schwenk, K.; Breslin, P.A.; Dunn, R.R. The evolution of sour taste. Proc. R. Soc. 2022, 289, 20211918.
- Mi, T.; Mack, J.O.; Koolmees, W.; Lyon, Q.; Yochimowitz, L.; Teng, Z.-Q.; Jiang, P.; Montell, C.; Zhang, Y.V. Alkaline taste sensation through the alkaliphile chloride channel in Drosophila. Nat. Metabol. 2023, 5, 466–480.
- Clark, J.T.; Ganguly, A.; Ejercito, J.; Luy, M.; Dahanukar, A.; Ray, A. Chemosensory detection of aversive concentrations of ammonia and basic volatile amines in insects. Iscience 2023, 26, 105777.
- Tian, L.; Zhang, H.; Yang, S.; Luo, A.; Kamau, P.M.; Hu, J.; Luo, L.; Lai, R. Vertebrate OTOP1 is also an alkali-activated channel. Nat. Commun. 2023, 14, 26.
- Delventhal, R.; Menuz, K.; Joseph, R.; Park, J.; Sun, J.; Carlson, J. The taste response to ammonia in Drosophila. Sci. Rep. 2017, 7, 43754.
- Min, S.; Ai, M.; Shin, S.A.; Suh, G.S. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc. Natl. Acad. Sci. USA 2013, 110, E1321–E1329.
- Kloehn, N.W.; Brogden, W. The alkaline taste: A comparison of absolute thresholds for sodium hydroxide on the tip and mid-dorsal surfaces of the tongue. Am. J. Psychol. 1948, 61, 90–93.
- Liljestrand, G.; Zotterman, Y. The alkaline taste. Acta Physiol. Scand. 1955, 35, 380–389.
- Bryant, B.P. Mechanisms of somatosensory neuronal sensitivity to alkaline pH. Chem. Senses 2005, 30, i196–i197.
- Beidler, L.M.; Gross, G.W. The nature of taste receptor sites. In Contributions to Sensory Physiology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 5, pp. 97–127.
- Taylor, N.W.; Farthing, F.R.; Berman, R. Quantitative measurements on the acid taste and their bearing on the nature of the nerve receptor. Protoplasma 1930, 10, 84–97.
- Gardner, R. Lipid solubility and the sourness of acids: Implications for models of the acid taste receptor. Chem. Senses 1980, 5, 185–194.
- Lyall, V.; Alam, R.I.; Phan, D.Q.; Ereso, G.L.; Phan, T.-H.T.; Malik, S.A.; Montrose, M.H.; Chu, S.; Heck, G.L.; Feldman, G.M. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am. J. Physiol. Cell Physiol. 2001, 281, C1005–C1013.
- Liman, E.R.; Kinnamon, S.C. Sour taste: Receptors, cells and circuits. Curr. Opin. Physiol. 2021, 20, 8–15.
- Ugawa, S.; Minami, Y.; Guo, W.; Saishin, Y.; Takatsuji, K.; Yamamoto, T.; Tohyama, M.; Shimada, S. Receptor that leaves a sour taste in the mouth. Nature 1998, 395, 555–556.
- Stevens, D.R.; Seifert, R.; Bufe, B.; Müller, F.; Kremmer, E.; Gauss, R.; Meyerhof, W.; Kaupp, U.B.; Lindemann, B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 2001, 413, 631–635.
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Tränkner, D.; Ryba, N.J.; Zuker, C.S. The cells and logic for mammalian sour taste detection. Nature 2006, 442, 934–938.
- Richter, T.A.; Dvoryanchikov, G.A.; Roper, S.D.; Chaudhari, N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J. Neurosci. 2004, 24, 4088–4091.
- Richter, T.; Caicedo, A.; Roper, S. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J. Physiol. 2003, 547, 475–483.
- Ishimaru, Y.; Inada, H.; Kubota, M.; Zhuang, H.; Tominaga, M.; Matsunami, H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 12569–12574.
- LopezJimenez, N.D.; Cavenagh, M.M.; Sainz, E.; Cruz-Ithier, M.A.; Battey, J.F.; Sullivan, S.L. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 2006, 98, 68–77.
- Horio, N.; Yoshida, R.; Yasumatsu, K.; Yanagawa, Y.; Ishimaru, Y.; Matsunami, H.; Ninomiya, Y. Sour taste responses in mice lacking PKD channels. PLoS ONE 2011, 6, e20007.
- Nelson, T.M.; LopezJimenez, N.D.; Tessarollo, L.; Inoue, M.; Bachmanov, A.A.; Sullivan, S.L. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem. Senses 2010, 35, 565–577.
- Tu, Y.-H.; Cooper, A.J.; Teng, B.; Chang, R.B.; Artiga, D.J.; Turner, H.N.; Mulhall, E.M.; Ye, W.; Smith, A.D.; Liman, E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 2018, 359, 1047–1050.
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour sensing from the tongue to the brain. Cell 2019, 179, 392–402.e315.
- Teng, B.; Wilson, C.E.; Tu, Y.-H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr. Biol. 2019, 29, 3647–3656.e3645.
- Chang, R.B.; Waters, H.; Liman, E.R. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22320–22325.
- Hughes, I.; Binkley, J.; Hurle, B.; Green, E.D.; Sidow, A.; Ornitz, D.M. Identification of the Otopetrin Domain, a conserved domain in vertebrate otopetrins and invertebrate otopetrin-like family members. BMC Evol. Biol. 2008, 8, 1–10.
- Charlu, S.; Wisotsky, Z.; Medina, A.; Dahanukar, A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 2013, 4, 2042.
- Dweck, H.K.; Talross, G.J.; Luo, Y.; Ebrahim, S.A.; Carlson, J.R. Ir56b is an atypical ionotropic receptor that underlies appetitive salt response in Drosophila. Curr. Biol. 2022, 32, 1776–1787.e1774.
- Öhrwall, H. Untersuchungen über den Geschmackssinn 1. Skand. Arch. Für Physiol. 1891, 2, 1–69.
- Henning, H. Physiologie und Psychologie des Geschmacks. Ergeb. Physiol. 1921, 19, 1–78.
- de la Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Reeh, P.W.; Lampert, A.; Fischer, M.J.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292.
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543.
- Wang, X.; Li, G.; Liu, J.; Liu, J.; Xu, X.S. TMC-1 mediates alkaline sensation in C. elegans through nociceptive neurons. Neuron 2016, 91, 146–154.
- Paje, F.; Mossakowski, D. pH-preferences and habitat selection in carabid beetles. Oecologia 1984, 64, 41–46.
- Liang, Z.; Wilson, C.E.; Teng, B.; Kinnamon, S.C.; Liman, E.R. The proton channel OTOP1 is a sensor for the taste of ammonium chloride. Nat. Commun. 2023, 14, 6194.
- Devineni, A.V.; Sun, B.; Zhukovskaya, A.; Axel, R. Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. Elife 2019, 8, e47677.
This entry is offline, you can click here to edit this entry!
