Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bacteriophages (or phages) are viruses that specifically infect and kill bacteria at the end of the phage infection cycle, causing bacterial cell death and lysis.
- multidrug-resistant (MDR) bacteria
- phage host range
- narrow host range
1. Introduction
Multidrug resistance (MDR) has become a growing public health concern globally as more microbes, including bacteria, have developed new resistance mechanisms to counter the antibiotics and chemotherapeutic agents designed to kill them. Over the past decade, the number of MDR organisms is reported to have increased dramatically. Moreover, antibiotics reportedly no longer work effectively on several species of common bacteria. These are termed ESKAPE pathogens, an acronym that encompasses Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter faecium [1]. Notably, mechanisms such as horizontal resistance gene transfer and multidrug efflux pump systems that pump antibiotics out of the cell can both enable acquired resistance in MDR organisms [2]. This development is associated with prolonged illnesses, increased medical costs, high mortality rates and foodborne disease outbreaks, making MDR a global interest and high-priority issue [3]. According to the World Health Organization (WHO), the main causes of antimicrobial resistance include the misuse or overuse of antibiotics; inadequate prevention and control; a lack of clean water, proper sanitation and hygiene; a lack of awareness and education; and finally, the inadequate enforcement of legislation [4].
The increasing threat posed by these MDR bacteria has prompted the re-emergence of bacteriophages (viruses that infect and kill bacteria specifically) as a potential alternative to antibiotic regimens [5]. In fact, the first recorded successful clinical use of bacteriophage therapy via intravenous route was conducted only quite recently, in 2016, at the University of California San Diego (United States, U.S.), where a phage was used to treat a severe MDR A. baumannii infection. Phage treatment has sparked increased public interest ever since that milestone. Phage banks and international phage directories have also been gradually developed to facilitate the conversion of phage research into clinical use [6]. As of now, phage therapy has been given emergency use authorization (EUA) by the U.S. Food and Drug Administration (FDA), which permits the compassionate use of this therapy depending on the severity of the patients’ cases. Patients with extensive MDR infections, such as cystic fibrosis, with underlying pulmonary infections where antibiotics do not work effectively, are often granted compassionate use [7].
2. Brief Overview of Bacteriophages and Their Interactions with Bacterial Hosts
Bacteriophages (or phages) are viruses that specifically infect and kill bacteria at the end of the phage infection cycle, causing bacterial cell death and lysis. In early 1896, a British bacteriologist, Ernest Hankin, noticed the presence of antibacterial activity in the Ganges and Jumna rivers (India). An unknown substance that was found to be heat-labile and could pass through very fine porcelain filters appeared to exhibit antibacterial properties, reducing the spread of cholera infection in villages [8]. Two years later, Nikolay Gamaleya, a Russian bacteriologist, noticed a similar event while studying Bacillus anthracis [9]. However, the “official” discovery of bacteriophages occurred in 1915 and 1917, when British pathologist Frederick Twort described a similar phenomenon while studying staphylococci and suggested that viruses might be responsible for this antibacterial activity [10], and French microbiologist Felix d’Herelle published a paper clearly defining the viral nature of the invisible anti-Shiga microbe and named it a “bacteriophage”—a combination of the words “bacteria” and “phagein” (which means to eat or devour in Greek) [11].
Like other viruses, bacteriophages are said to be obligate intracellular parasites. Despite having the genetic material to direct replication, they have to rely on the reproductive machinery of the bacterial host. Generally, each phage consists of a single type of nucleic acid in the head, either double- or single-stranded deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), which is circular or linear [12]. The size of the genome that determines all the properties of the phage can range from a few to hundreds of kilobases, and they are enclosed within a protein coat—a capsid [13].
In 1967, Bradley classified phages into six basic types (Groups A to F) based on their morphological characteristics and nucleic acid composition: filamentous phages, icosahedral phages, and tailed phages, with ssRNA or ssDNA [14]. According to Bradley, Group A phages have long tails with a contractile sheath; Group B and C phages have long and short non-contractile tails, respectively; Group D and E phages lack a tail, but the former have big capsomeres and the latter have small capsomeres; and Group F phages are filamentous, with flexible filaments. This classification scheme still serves as the basis for the current taxonomy of phages. In 1995, the International Committee on the Taxonomy of Viruses (ICTV) classified tailed phages into three families: Myoviridae, Siphoviridae and Podoviridae, corresponding to Groups A, B and C of Bradley’s classification [15]. Phages were then classified by the ICTV into one major order, 19 families, and 31 genera [16]. It is worth mentioning that most of the phages (96%) identified were tailed dsDNA phages belonging to the families Myoviridae, Podoviridae or Siphoviridae of the same order Caudovirales, while only 3.6% of such phages were polyhedral, filamentous, or pleomorphic [17].
2.1. Phage Life Cycle and Its Relations to Phage Host Range
Phage host ranges are determined by their ability to replicate in and kill their bacterial host. Therefore, it is vital to study all aspects of the phage-host interactions, not just the attachment, so as to achieve a greater understanding of the issues of broad and narrow host ranges. After the phage binds to the host bacteria, it will initiate different phage–host interactions, namely, the lytic cycle, lysogenic cycle, pseudolysogenic cycle and chronic cycle [18]. Virulent phages usually take control of the host metabolism after infection and use it for replication and synthesizing new phage particles. The release of viral progeny from the host cell by lysis will result in a new lytic cycle carried out by the viral progeny. On the other hand, the lysogenic cycle involves the integration of the viral nucleic acid into the bacterial genome, forming a prophage. Together with the bacterial genome, these prophages are transmitted to the host descendants until the lytic cycle is induced. A pseudolysogenic cycle is a variant of the lysogenic cycle where the phage is inactivated within the host. This usually occurs during starvation. Finally, in the chronic cycle, phages replicate in the host and exit the host cell by budding instead of by lysis, thus sparing the host and resulting in continuous phage production. The above phage–host interaction is briefly summarized in Figure 1.
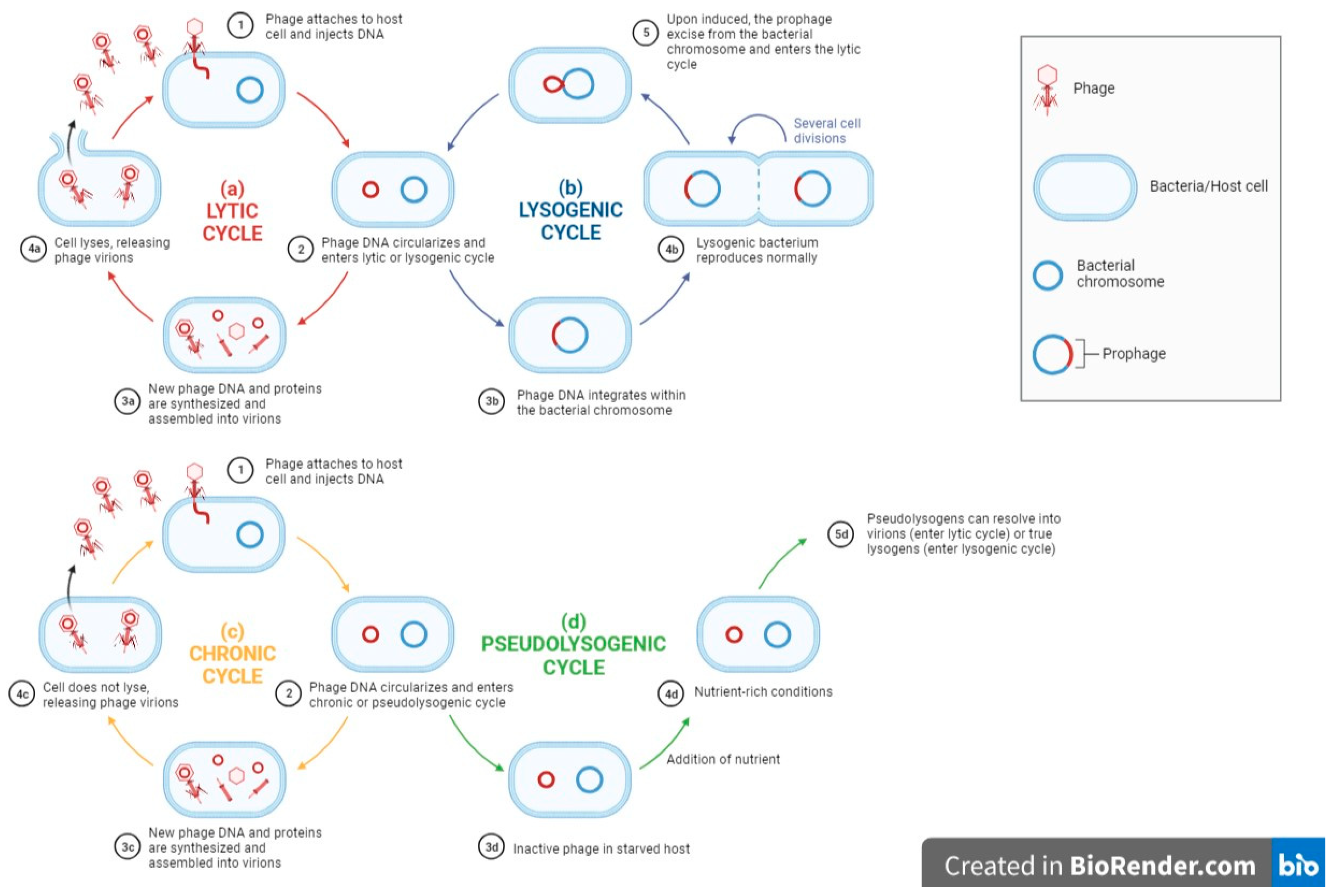
Figure 1. A flow diagram showing general phage–host interactions (a) lytic cycle, (b) chronic cycle, (c) lysogenic cycle, (d) pseudolysogenic cycle. Adapted from “Lytic and Lysogenic Cycle”, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates (accessed on 23 May 2023).
The attachment stage in the cycles summarized above is often the focus in studies of the molecular mechanisms of broad-host range phages (see below). However, following adsorption, the phage must go on to replicate successfully in the host, which may depend upon the phages’ genetics and regulatory mechanisms. In addition, host range modulation may also largely depend on the different proteins present, as well as the host receptor-binding proteins or RBPs adaptations.
2.2. Bacteriophage Adsorption and Receptors Present in MDR Bacteria
Commonly, the first stage of both lytic and temperate infections begins with adsorption. This involves the specific phage–host interaction between the RBPs or adhesins of a phage and receptors on the bacterial cell surface [19]. Tailed phages use their tail adhesins (including base plate proteins and tail fibers) to interact with bacterial receptors and thus penetrate the bacteria cell surface to eject phage DNA, whereas tail-less phages use capsid proteins such as spike protein G, capsid protein F, and DNA pilot protein H to carry out the tail-associated function to enable subsequent DNA ejection [20]. In tailed phages, there are three steps involved in phage adsorption: initial contact with bacterial surface receptors, followed by reversible binding, and finally irreversible binding, during which the phages can no longer dissociate from the bacterial cell surface [21][22]. The host specificity of the phages is determined at this stage because the subsequent steps in the lytic cycle can only proceed if the phage successfully attaches to and penetrates the bacterial cell; the identification of cell surface receptors is therefore crucial. There are various cell surface receptors on Gram-positive bacteria and Gram-negative bacteria that can be targeted by the phage during adsorption. These are either constituents of bacterial cell walls such as proteins, polysaccharides, lipopolysaccharides, and carbohydrates, or protruding structures such as pili, flagella, and capsules [23].
In Gram-positive bacteria, the common surface receptors that can be recognized by the phage are on the one hand peptidoglycan or murein, and on the other teichoic acid, both important components of the bacterial cell wall [22]. In MDR Gram-positive strains such as MRSA, meanwhile, studies have reported that the wall teichoic acid acts as the receptor for broad-host range phage ΦMR003 to recognize and bind [24]. Another phage, ΦSA039, that can infect MRSA also recognizes wall teichoic acid as the receptor, though with the difference that ΦSA039 also requires the β-GlcNAc residue on wall teichoic acid for phage adsorption [25]. In addition, the membrane proteins PIPEF (phage infection protein of Enterococcus faecalis) and enterococcal polysaccharide antigen (Epa) have been identified as receptors of Vancomycin-resistant enterococci (VRE) and can be attached by a collection of enterococcus-specific phages [26].
In Gram-negative bacteria, the typical surface receptors that can be attached by the phage are lipopolysaccharides and proteins located on the outer membrane, flagella, pili and capsules [22]. In the MDR Gram-negative Shigella species, it was recently discovered that a phage called Sfin-1 can infect Shigella flexneri, Shigella sonnei and Shigella dysenteriae by recognizing a lipopolysaccharide O-antigen for adsorption [27]. Moreover, capsular polysaccharides consisting of tightly packed repeating K units have been identified as the receptors of carbapenem-resistant A. baumanni for the A. baumannii-specific phage øCO01 to adsorb [28]. Furthermore, in a personalized nebulized phage therapy study, two Pseudomonas phages LPS-5 and TIVP-H6 were shown to bind to lipopolysaccharides and type-IV pili, respectively, of MDR P. aeruginosa [29]. Finally, the phages Pharr and ΦKpNIH-2 have been shown to infect MDR K. pneumoniae by binding to their capsular polysaccharides and outer membrane porin OmpC or lipopolysaccharides, respectively [30].
In brief, the potential of a phage to affect a bacterial population and the susceptibility of a bacterium to phage infection largely depends on the host range of the phage, which in turn depends primarily on its adsorption properties [31]. Both bacterial and phage characteristics, such as the nature and location of bacterial cell surface receptors and RBPs, can limit the host range of phages, making them specific to one bacterial species or even to only a few strains within a bacterial species. Before starting phage therapy, phage typing or applying phages in the food industry, it is therefore crucial to identify their specificity range against the pathogenic bacteria in question.
2.3. Phage–Host Interaction
As mentioned earlier, phages can kill antibiotic-resistant bacteria in the final stage of the infection cycle. The life cycle of phages determines the role they play and how they can be applied in different approaches. There are two main modes of infection by bacteriophages—lytic (or virulent) infection and temperate infection, carried out by lytic and temperate phages, respectively. In lytic infection, phages infect and kill their bacterial host cell in four stages: adsorption on host cell surface, insertion of phage DNA, replication of phage DNA, lysis of host cell, and releasing of newly formed phages into the environment [32]. In contrast, there are two possible outcomes in a temperate infection: either the host cell lyses and releases newly formed phages, similar to a lytic infection, or the phage DNA may integrate into the bacterial chromosome to become a prophage, a process known as lysogeny [33]. This prophage is non-infectious until it is induced by UV irradiation or chemicals, inducing it to enter a lytic cycle [34]. The key thing to note here is that phage induction is primarily caused by DNA damage. Another potential outcome of lysogeny is lysogenic conversion, i.e., the alteration of bacterial phenotypes and behaviors with virulence or other determinants such as toxin genes (usually acquired from temperate phages). These latter may protect bacteria from phage reinfection [35]. Therefore, lytic phages are usually the first choice for phage therapy, while the use of temperate phages is much less common.
However, Edgar et al. (2012) attempted to use phage-based delivery systems, where temperate phages were used as vehicles to deliver DNA encoding drug-sensitizing genes to bacteria, so as to increase bacterial susceptibility to antibiotics [36]. They were successful in doing this: streptomycin- and nalidixic acid (NA)-resistant E. coli K-12 lysogenized with designed phages carrying the genes rpsL and gyrA (confer sensitivity to streptomycin and NA, respectively) showed restored antibiotic sensitivity. Additionally, Park et al. (2017) integrated the CRISPR/Cas9 system into the genome of a temperate phage (φ SaBov) in the hope of improving the current limitations of phage-based delivery systems [37]. Besides managing to expand the host specificity by complementing the tail fiber protein of the phage, they also managed to remove the virulence factor genes from the S. aureus strain (RF122) to prevent contamination and the spread of virulence genes by transduction. The 10 superantigens (e.g., sec, seg and selo) and 11 cytolysins (e.g., hla, hlgA and lukD) of RF122 were removed from the chromosome of S. aureus using allelic exchange by a modified shuttle vector system (pMAD-secY system). The results show that the phage lysates generated from this modified strain (R122-19Δnuc) did not cause any cytotoxicity or superantigenicity [37].
These experiments suggest that genetically engineered temperate phages may be used as gene delivery vehicles to create programmable gene-specific antimicrobials that are less likely to drive resistance than antibiotics. However, not much is known about the potential limitations of this study—for example, whether such alterations of bacterial genes might alter microbiological niches in the environment.
This entry is adapted from the peer-reviewed paper 10.3390/ph16101467
References
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542.
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501.
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340.
- World Health Organization. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=The%20main%20drivers%20of%20antimicrobial,access%20to%20quality%2C%20affordable%20medicines%2C (accessed on 6 May 2023).
- Paul, V.D.; Sundarrajan, S.; Rajagopalan, S.S.; Hariharan, S.; Kempashanaiah, N.; Padmanabhan, S.; Sriram, B.; Ramachandran, J. Lysis-deficient phages as novel therapeutic agents for controlling bacterial infection. BMC Microbiol. 2011, 11, 195.
- Nagel, T.; Musila, L.; Muthoni, M.; Nikolich, M.; Nakavuma, J.L.; Clokie, M.R. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr. Opin. Virol. 2022, 53, 101208.
- Hitchcock, N.M.; Nunes, D.D.G.; Shiach, J.; Hodel, K.V.S.; Barbosa, J.D.V.; Rodrigues, L.A.P.; Coler, B.S.; Soares, M.B.P.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020.
- Hankin, M.E. The bactericidal action of the waters of the Jamuna and Ganges rivers on Cholera microbes. Ann. Inst. Pasteur 10:511–523 (1896). Bacteriophage 2011, 1, 117–126.
- Schullian, D.M.; Rogers, F.B. Notes and Events. J. Hist. Med. Allied Sci. 1979, XXXIV, 460–462.
- Twort, F. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243.
- Service, P. On an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. Res. Microbiol. 2007, 158, 553–554.
- Moineau, S. Bacteriophage; Elsevier EBooks: Amsterdam, The Netherlands, 2013; pp. 280–283.
- Zrelovs, N.; Dislers, A.; Kazaks, A. Motley Crew: Overview of the Currently Available Phage Diversity. Front. Microbiol. 2020, 11, 579452.
- E Bradley, D. Ultrastructure of bacteriophage and bacteriocins. Bacteriol. Rev. 1967, 31, 230–314.
- Ackermann, H. Tailed Bacteriophages: The Order Caudovirales. Adv. Virus Res. 1998, 51, 135–201.
- Ackermann, H.W. Classification of bacteriophages. Bacteriophages 2006, 2, 8–16.
- Ackermann, H.-W. Bacteriophage observations and evolution. Res. Microbiol. 2003, 154, 245–251.
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; De Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493.
- Le, S.; He, X.; Tan, Y.; Huang, G.; Zhang, L.; Lux, R.; Shi, W.; Hu, F. Mapping the Tail Fiber as the Receptor Binding Protein Responsible for Differential Host Specificity of Pseudomonas aeruginosa Bacteriophages PaP1 and JG004. PLoS ONE 2013, 8, e68562.
- Sun, Y.; Roznowski, A.P.; Tokuda, J.M.; Klose, T.; Mauney, A.; Pollack, L.; Fane, B.A.; Rossmann, M.G. Structural changes of tailless bacteriophage ΦX174 during penetration of bacterial cell walls. Proc. Natl. Acad. Sci. USA 2017, 114, 13708–13713.
- Storms, Z.J.; Sauvageau, D. Modeling tailed bacteriophage adsorption: Insight into mechanisms. Virology 2015, 485, 355–362.
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002.
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophage Receptors, Mechanisms of Phage Adsorption and Penetration into Host Cell. Pol. J. Microbiol. 2010, 59, 145–155.
- Peng, C.; Hanawa, T.; Azam, A.H.; LeBlanc, C.; Ung, P.; Matsuda, T.; Onishi, H.; Miyanaga, K.; Tanji, Y. Silviavirus phage ΦMR003 displays a broad host range against methicillin-resistant Staphylococcus aureus of human origin. Appl. Microbiol. Biotechnol. 2019, 103, 7751–7765.
- Azam, A.H.; Hoshiga, F.; Takeuchi, I.; Miyanaga, K.; Tanji, Y. Analysis of phage resistance in Staphylococcus aureus SA003 reveals different binding mechanisms for the closely related Twort-like phages ΦSA012 and ΦSA039. Appl. Microbiol. Biotechnol. 2018, 102, 8963–8977.
- Chatterjee, A.; Johnson, C.N.; Luong, P.; Hullahalli, K.; McBride, S.W.; Schubert, A.M.; Palmer, K.L.; Carlson, P.E., Jr.; Duerkop, B.A. Bacteriophage Resistance Alters Antibiotic-Mediated Intestinal Expansion of Enterococci. Infect. Immun. 2019, 87, e00085-19.
- Ahamed, S.T.; Roy, B.; Basu, U.; Dutta, S.; Ghosh, A.N.; Bandyopadhyay, B.; Giri, N. Genomic and Proteomic Characterizations of Sfin-1, a Novel Lytic Phage Infecting Multidrug-Resistant Shigella spp. and Escherichia coli C. Front. Microbiol. 2019, 10, 1876.
- Altamirano, F.G.; Forsyth, J.H.; Patwa, R.; Kostoulias, X.; Trim, M.; Subedi, V.O.R.I.P.; Archer, S.; Morris, V.O.R.I.P.C.; Oliveira, C.; Kielty, L.; et al. Bacteriophages targeting Acinetobacter baumannii capsule induce antimicrobial resensitization. bioRxiv 2020, 1–28.
- Chan, B.; Stanley, G.L.; Kortright, K.E.; Modak, M.; Ott, I.M.; Sun, Y.; Würstle, S.; Grun, C.; Kazmierczak, B.; Rajagopalan, G.; et al. Personalized Inhaled Bacteriophage Therapy Decreases Multidrug-Resistant Pseudomonas aeruginosa. medRxiv 2023.
- Hesse, S.; Rajaure, M.; Wall, E.; Johnson, J.; Bliskovsky, V.; Gottesman, S.; Adhya, S. Phage Resistance in Multidrug-Resistant Klebsiella pneumoniae ST258 Evolves via Diverse Mutations That Culminate in Impaired Adsorption. mBio 2020, 11, e02530-19.
- Abedon, S.; Thomas-Abedon, C. Phage Therapy Pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47.
- Kutter, E.; Raya, R.; Carlson, K. Molecular Mechanisms of Phage Infection. In Bacteriophages: Biology and Application; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 165–222.
- Little, J.W. Lysogeny, Prophage Induction, and Lysogenic Conversion. Phages 2014, 37–54.
- Skorb, E.V.; Andreeva, D.V.; Raiski, A.P.; Belyasova, N.A.; Möhwald, H.; Sviridov, D.V. Titanium dioxide-assisted photocatalytic induction of prophages to lytic cycle. Photochem. Photobiol. Sci. 2011, 10, 1974–1978.
- Moons, P.; Faster, D.; Aertsen, A. Lysogenic Conversion and Phage Resistance Development in Phage Exposed Escherichia coli Biofilms. Viruses 2013, 5, 150–161.
- Edgar, R.; Friedman, N.; Molshanski-Mor, S.; Qimron, U. Reversing Bacterial Resistance to Antibiotics by Phage-Mediated Delivery of Dominant Sensitive Genes. Appl. Environ. Microbiol. 2012, 78, 744–751.
- Park, J.Y.; Moon, B.Y.; Park, J.W.; Thornton, J.A.; Park, Y.H.; Seo, K.S. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus. Sci. Rep. 2017, 7, srep44929.
This entry is offline, you can click here to edit this entry!
