Shiga-toxin-producing Escherichia coli (STEC) is typically detected on food products, mainly due to cross-contamination with faecal matter. The serotype O157:H7 has been of major public health concern due to the severity of illness caused, prevalence, and management. In the food chain, the main methods of controlling contamination by foodborne pathogens often involve the application of antimicrobial agents, which are now becoming less efficient. There is a growing need for the development of new approaches to combat these pathogens, especially those that harbour antimicrobial resistant and virulent determinants. Strategies to also limit their presence on food contact surfaces and food matrices are needed to prevent their transmission. Studies have revealed that bacteriophages are useful non-antibiotic options for biocontrol of E. coli O157:H7 in both animals and humans. Phage biocontrol can significantly reduce E. coli O157:H7, thereby improving food safety. However, before being certified as potential biocontrol agents, the safety of the phage candidates must be resolved to satisfy regulatory standards, particularly regarding phage resistance, antigenic properties, and toxigenic properties.
- food borne infection
- antimicrobial resistance
- Escherichia coli O157:H7
- phage thera
1. Introduction
2. Evolution, Virulence and Pathogenicity of E. coli O157:H7
2.1. Transmission of E. coli O157:H7 to Humans and Animals
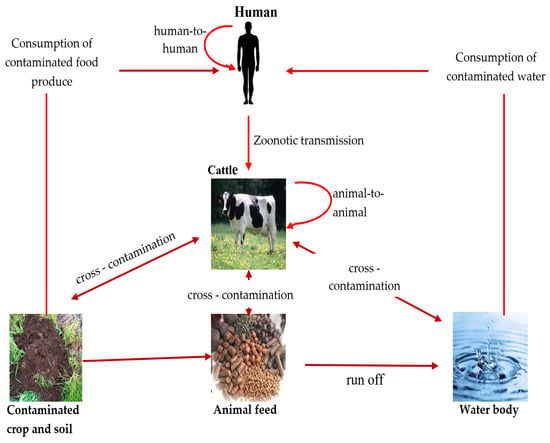
2.1.1. Contaminated Food as a Transmission Vector
2.1.2. Contaminated Water as a Transmission Vector
2.1.3. Person-To-Person Transmission
2.2. Epidemiology of E. coli O157:H7
2.3. Treatment for E. coli O157:H7 Infections and Antimicrobial Resistance
3. Bacteriophages
4. Challenges Faced in the Application of E. coli O157:H7 Phages
Phage Resistance by E. coli O157:H7
5. Determining the Safety of E. coli O157:H7 Specific Bacteriophages as a Biocontrol Agent
6. Conclusions
In the current era of antimicrobial resistance and the pursuit of alternative methods to combat E. coli O157:H7 infections, there has been a renewed interest in phage research. This is evident in the accumulated evidence suggesting that phage application is an effective method of biocontrol of foodborne bacterial pathogens on fresh produce and other foods. Phages has been used as a natural antimicrobial method to reduce E. coli O157:H7 from the food supply. Phage-based medications have been approved by the Food and Drug Administration (FDA) in certain countries, such as the United States, Canada, and Israel for the treatment of E. coli strains, including the use of various phage cocktails. Numerous studies have also produced novel phages and showed their effectiveness in inhibiting the growth of E. coli O157:H7 in food products without changing the organoleptic properties. They have also been proven to reduce E. coli O157:H7 in vivo through the use of mice. Ongoing research involves the construction of phage libraries, thereby enabling the use of phage cocktails to inhibit phage mutant strains, increase host range, and increase infectivity. Nevertheless, the European Union remains concerned about the utilization of these products due to the limited availability of safety data and comprehensive research on the potential consequences of phage discharge into the environment. One of the most important aspects of creating One Health approaches to lowering health risks for people, agricultural systems, and natural ecosystems is the effective use of “safe” phages as a biocontrol agent in combating pathogens like E. coli O157:H7 and resolving the issue of antibiotic resistance in preharvest livestock environments. In order to advance the development of novel antibacterial strategies, there is a need to conduct further investigation into the phage properties that make them safe and suitable as a biocontrol and the underlying mechanism through which phages can effectively inhibit the expression of host genes. The utilization of advanced technologies like WGS to carefully scrutinize and characterize phage genomes presents a solution to this concern. This, in turn, will improve both patient care and infection control strategies, ultimately leading to a decrease in the incidence of severe E. coli O157:H7 infections.
This entry is adapted from the peer-reviewed paper 10.3390/foods12213989
References
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.; Naas, T.; Carattoli, A.; Martínez-Medina, M. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463.
- Farrokh, C.; Jordan, K.; Auvray, F.; Glass, K.; Oppegaard, H.; Raynaud, S.; Thevenot, D.; Condron, R.; De Reu, K.; Govaris, A. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food. Microbiol. 2013, 162, 190–212.
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76.
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140.
- Akindolire, M.A.; Ateba, C.N. Use of pulsed field gel electrophoresis genetic typing for tracing contamination with virulent Escherichia coli O157: H7 in beef-cattle producing farms. Gene Rep. 2018, 13, 59–65.
- Sugiyama, A.; Iwade, Y.; Akachi, S.; Nakano, Y.; Matsuno, Y.; Yano, T.; Yamauchi, A.; Nakayama, O.; Sakai, H.; Yamamoto, K.-i. An outbreak of Shigatoxin-producing Escherichia coli O157: H7 in a nursery school in Mie Prefecture. Jpn. J. Infect. Dis. 2005, 58, 398.
- Shen, J.; Rump, L.; Ju, W.; Shao, J.; Zhao, S.; Brown, E.; Meng, J. Virulence characterization of non-O157 Shiga toxin-producing Escherichia coli isolates from food, humans and animals. Food Microb. 2015, 50, 20–27.
- Ateba, C.N.; Mbewe, M. Detection of Escherichia coli O157: H7 virulence genes in isolates from beef, pork, water, human and animal species in the northwest province, South Africa: Public health implications. Res. Microbiol. 2011, 162, 240–248.
- Ateba, C.N.; Mbewe, M. Genotypic characterization of Escherichia coli O157: H7 isolates from different sources in the north-west province, South Africa, using enterobacterial repetitive intergenic consensus PCR analysis. Int. J. Mol. Sci. 2014, 15, 9735–9747.
- Law, D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J. Appl. Microb. 2000, 88, 729–745.
- Rivera-Betancourt, M.; Shackelford, S.D.; Arthur, T.M.; Westmoreland, K.E.; Bellinger, G.; Rossman, M.; Reagan, J.O.; Koohmaraie, M. Prevalence of Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella in two geographically distant commercial beef processing plants in the United States. J. Food Protect. 2004, 67, 295–302.
- Shu, X.; Singh, M.; Karampudi, N.B.R.; Bridges, D.F.; Kitazumi, A.; Wu, V.C.; De los Reyes, B.G. Responses of Escherichia coli and Listeria monocytogenes to ozone treatment on non-host tomato: Efficacy of intervention and evidence of induced acclimation. PLoS ONE 2021, 16, e0256324.
- Liu, D.; Lawrence, M.L.; Ainsworth, A.J.; Austin, F.W. Comparative assessment of acid, alkali and salt tolerance in Listeria monocytogenes virulent and avirulent strains. FEMS Microb. Lett. 2005, 243, 373–378.
- Bridges, D.F.; Rane, B.; Wu, V.C. The effectiveness of closed-circulation gaseous chlorine dioxide or ozone treatment against bacterial pathogens on produce. Food Cont. 2018, 91, 261–267.
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 8 August 2023).
- Gould, L.H.; Mody, R.K.; Ong, K.L.; Clogher, P.; Cronquist, A.B.; Garman, K.N.; Lathrop, S.; Medus, C.; Spina, N.L.; Webb, T.H. Increased recognition of non-O157 Shiga toxin–producing Escherichia coli infections in the United States during 2000–2010: Epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog. Dis. 2013, 10, 453–460.
- Karlsson, M.E.; Uhlig, E.; Håkansson, Å.; Alsanius, B.W. Seed inoculation with antagonistic bacteria limits occurrence of E. coli O157: H7gfp+ on baby spinach leaves. BMC Microbiol. 2022, 22, 131.
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493.
- Griffin, P. Escherichia coli O157: H7 and other enterohemorrhagic Escherichia coli. Infect. Gas. Tract. 1995, 169, 739–761.
- Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. Antibiotics 2021, 10, 1342.
- Huchin, C.; Briceño, M.A.; Mendoza, T.; Martínez, A.P.; Ramírez, M.A.; Torres, J.C. Prevalence and drug-resistance patterns of Enterotoxigenic Escherichia coli and Shigella species among children with diarrhea in Merida City, Mexico. J. Biosci. Med. 2017, 6, 22–33.
- Arbab, S.; Ullah, H.; Wang, W.; Zhang, J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786.
- Gambushe, S.M.; Zishiri, O.T.; El Zowalaty, M.E. Review of Escherichia coli O157: H7 prevalence, pathogenicity, heavy metal and antimicrobial resistance, African perspective. Infect. Drug Resist. 2022, 15, 4645–4673.
- Law, D. The history and evolution of Escherichia coli O157 and other Shiga toxin-producing E. coli. World J. Microbiol. 2000, 16, 701–709.
- Wick, L.M.; Qi, W.; Lacher, D.W.; Whittam, T.S. Evolution of genomic content in the stepwise emergence of Escherichia coli O157: H7. J. Bacteriol. 2005, 187, 1783–1791.
- Feng, P.; Lampel, K.A.; Karch, H.; Whittam, T.S. Genotypic and phenotypic changes in the emergence of Escherichia coli O157: H7. J. Infect. Dis. 1998, 177, 1750–1753.
- Idland, L.; Bø-Granquist, E.G.; Aspholm, M.; Lindbäck, T. The Ability of Shiga Toxin-Producing Escherichia coli to Grow in Raw Cow’s Milk Stored at Low Temperatures. Foods 2022, 11, 3411.
- Al-Aalim, A.M.A. Immune Responses Induced by Standard and Extracted Lipopolysaccharide (LPS) from Escherichia coli. Ph.D. Thesis, University of Basrah, Basrah, Iraq, 2022.
- Gebisa, E.S.; Gerasu, M.A.; Leggese, D.T. A Review on Virulence Factors of Escherichia coli. Vet. Anim. Sci. 2019, 7, 83–93.
- Karch, H.; Bielaszewska, M. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157: H−strains: Epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J. Clin. Micobiol. 2001, 39, 2043–2049.
- Monday, S.R.; Minnich, S.A.; Feng, P.C. A 12-base-pair deletion in the flagellar master control gene flhC causes nonmotility of the pathogenic German sorbitol-fermenting Escherichia coli O157: H−strains. J. Bacteriol. 2004, 186, 2319–2327.
- Lim, J.Y.; Yoon, J.W.; Hovde, C.J. A brief overview of Escherichia coli O157: H7 and its plasmid. J. Microbiol. Biotechnol. 2010, 20, 5.
- Stanton, E. The Impact of Mobile Genetic Elements as Drivers of Genome Diversification in Bovine Escherichia coli O157: H7; The University of Wisconsin-Madison: Madison, WI, USA, 2020.
- Nguyen, T.T.H.; Kikuchi, T.; Tokunaga, T.; Iyoda, S.; Iguchi, A. Diversity of the tellurite resistance gene operon in Escherichia coli. Front. Microbiol. 2021, 12, 681175.
- Lee, M.-S.; Tesh, V.L. Roles of Shiga toxins in immunopathology. J. Toxins 2019, 11, 212.
- Rodríguez-Rubio, L.; Haarmann, N.; Schwidder, M.; Muniesa, M.; Schmidt, H. Bacteriophages of Shiga toxin-producing Escherichia coli and their contribution to pathogenicity. J. Pathog. 2021, 10, 404.
- Verma, A.; Bolton, F.; Fiefield, D.; Lamb, P.; Woloschin, E.; Smith, N.; McCann, R. An outbreak of E. coli O157 associated with a swimming pool: An unusual vehicle of transmission. Epidemiol. Infect. 2007, 135, 989–992.
- Ababu, A.; Endashaw, D.; Fesseha, H. Isolation and antimicrobial susceptibility profile of Escherichia coli O157: H7 from raw milk of dairy cattle in Holeta district, Central Ethiopia. Int. J. Microbiol. 2020, 2020, 6626488.
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W., IV. Shiga toxin-producing Escherichia coli. Adv. Appl. Microbiol. 2014, 86, 145–197.
- Montso, P.K.; Mlambo, V.; Ateba, C.N. The first isolation and molecular characterization of Shiga Toxin-producing virulent multi-drug resistant atypical enteropathogenic Escherichia coli O177 serogroup from South African Cattle. Front. Cell. Infect. Microbiol. 2019, 9, 333.
- Angelopoulou, M.; Petrou, P.; Misiakos, K.; Raptis, I.; Kakabakos, S. Simultaneous Detection of Salmonella typhimurium and Escherichia coli O157: H7 in Drinking Water and Milk with Mach–Zehnder Interferometers Monolithically Integrated on Silicon Chips. Biosensors 2022, 12, 507.
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struc. Biol. 2013, 23, 669–677.
- Xie, Y.; Zhu, L.; Lyu, G.; Lu, L.; Ma, J.; Ma, J. Persistence of E. coli O157: H7 in urban recreational waters from Spring and Autumn: A comparison analysis. Environ. Sci. Pollut. Res. 2022, 29, 39088–39101.
- Iwu, C.D.; du Plessis, E.; Korsten, L.; Okoh, A.I. Antibiogram imprints of E. coli O157: H7 recovered from irrigation water and agricultural soil samples collected from two district municipalities in South Africa. Int. J. Environ. Stud. 2021, 78, 940–953.
- Odo, S.E.; Uchechukwu, C.F.; Ezemadu, U.R. Foodborne Diseases and Intoxication in Nigeria: Prevalence of Escherichia coli 0157: H7, Salmonella, Shigella and Staphylococcus aureus. J. Adv. Microbiol. 2021, 20, 84–94.
- Lopes, F.A.; Davies-Colley, R.; Piazi, J.; Silveira, J.S.; Leite, A.C.; Lopes, N.I.A. Challenges for contact recreation in a tropical urban lake: Assessment by a water quality index. Environ. Dev. Sustain. 2020, 22, 5409–5423.
- Chávez-Díaz, L.V.; Gutiérrez-Cacciabue, D.; Poma, H.R.; Rajal, V.B. Sediments quality must be considered when evaluating freshwater aquatic environments used for recreational activities. Int. J. Hyg. Environ. Health 2020, 223, 159–170.
- Akindolire, M.A. Molecular Characterisation of Escherichia coli O157: H7 Specific Bacteriophages from Cattle Faeces. Ph.D. Thesis, North-West University (South Africa), Mahikeng, South Africa, 2019.
- Seabela, M.D.L. Assessment of Food Safety Hazards among Day Care Centres in Mbombela, Republic of South Africa. Master’s Dissertation, University of Johannesburg, Johannesburg, South Africa, 2020.
- Barkley, J.; Viveiros, B.; Michael Gosciminski, M.; Utpala Bandy, M. Preventing foodborne and enteric illnesses among at-risk populations in the United States and Rhode Island. Rhode Isl. Med. J. 2016, 99, 25.
- Mesele, F.; Abunna, F. Escherichia coli O157: H7 in foods of animal origin and its food safety implications. Adv. Biol. Res. 2019, 13, 134–145.
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157: H7 outbreaks, united states, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603.
- Lupindua, A.M. Epidemiology of Shiga toxin-producing Escherichia coli O157: H7 in Africa in review. S. Afr. J. Infect. Dis. 2018, 33, 24–30.
- Browning, N.; Botha, J.; Sacho, H.; Moore, P. Escherichia coli O157: H7 haemorrhagic colitis. Report of the first South African case. S. Afr. J. Surg. 1990, 28, 28–29.
- Germani, Y.; Soro, B.; Vohito, M.; Morel, O.; Morvan, J. Enterohaemorrhagic Escherichia coli in Central African Republic. Lancet 1997, 349, 1670.
- Raji, M.; Minga, U.; Machang’u, R. Prevalence and characterization of verotocytoxin producing Escherichia coli O157 from diarrhoea patients in Morogoro, Tanzania. Tanzan. J. Health Res. 2008, 10, 151–158.
- WHO. The Fight against Antimicrobial Resistance Is Closely Linked to the Sustainable Development Goals. Available online: https://apps.who.int/iris/bitstream/handle/10665/337519/WHO-EURO-2020-1634-41385-56394-eng.pdf (accessed on 26 December 2022).
- Ackermann, H.-W.; Martin, M.; Vieu, J.-F.; Nicolle, P. Félix d’Hérelle: His life and work and the foundation of a bacteriophage reference center. ASM News 1982, 48, 347–348.
- Aswani, V.H.; Shukla, S.K. An Early History of Phage Therapy in the United States: Is it Time to Reconsider? J. Clin. Med. Res. 2021, 19, 82–89.
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45.
- Fernández, L.; Rodríguez, A.; García, P. Phage or foe: An insight into the impact of viral predation on microbial communities. ISME J. 2018, 12, 1171–1179.
- Melo, L.D.; Oliveira, H.; Santos, S.B.; Sillankorva, S.; Azeredo, J. Phages against infectious diseases. In Bioprospecting; Springer: Berlin/Heidelberg, Germany, 2017; pp. 269–294.
- d’Herelle, F. An invisible microbe that is antagonistic to the dysentery Bacillus. CR Acad. Sci. 1917, 165, 373–375.
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31.
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235.
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179.
- Pinto, G.; Almeida, C.; Azeredo, J. Bacteriophages to control Shiga toxin-producing E. coli—Safety and regulatory challenges. Crit. Rev. Biotechnol. 2020, 40, 1081–1097.
- Dennehy, J.J.; Abedon, S.T. Adsorption: Phage acquisition of bacteria. In Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2021; pp. 93–117.
- Lopez, M.E.S.; Gontijo, M.T.P.; Cardoso, R.R.; Batalha, L.S.; Eller, M.R.; Bazzolli, D.M.S.; Vidigal, P.M.P.; Mendonça, R.C.S. Complete genome analysis of Tequatrovirus ufvareg1, a Tequatrovirus species inhibiting Escherichia coli O157: H7. Front. Cell. Infect. Microbiol. 2023, 13, 560.
- Zhu, W.; Ding, Y.; Huang, C.; Wang, J.; Wang, J.; Wang, X. Genomic characterization of a novel bacteriophage STP55 revealed its prominent capacity in disrupting the dual-species biofilm formed by Salmonella Typhimurium and Escherichia coli O157: H7 strains. Arch. Microbiol. 2022, 204, 597.
- Osman, A.-H.; Kotey, F.C.; Odoom, A.; Darkwah, S.; Yeboah, R.K.; Dayie, N.T.; Donkor, E.S. The Potential of Bacteriophage-Antibiotic Combination Therapy in Treating Infections with Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 1329.
- Zhang, Y.; Huang, H.-H.; Ma, L.Z.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Inactivation of mixed Escherichia coli O157: H7 biofilms on lettuce by bacteriophage in combination with slightly acidic hypochlorous water (SAHW) and mild heat treatment. Food Microbiol. 2022, 104, 104010.
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463.
- Rohde, C.; Resch, G.; Pirnay, J.-P.; Blasdel, B.G.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E. Expert opinion on three phage therapy related topics: Bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 2018, 10, 178.
- Simmons, E.L.; Drescher, K.; Nadell, C.D.; Bucci, V. Phage mobility is a core determinant of phage–bacteria coexistence in biofilms. ISME J. 2018, 12, 531–543.
- Rostøl, J.T.; Marraffini, L. (Ph) ighting phages: How bacteria resist their parasites. Cell Host Microbe 2019, 25, 184–194.
- Tomat, D.; Quiberoni, A.; Mercanti, D.; Balagué, C. Hard surfaces decontamination of enteropathogenic and Shiga toxin-producing Escherichia coli using bacteriophages. Food Res. Int. 2014, 57, 123–129.
- Fu, Q.; Li, S.; Wang, Z.; Shan, W.; Ma, J.; Cheng, Y.; Wang, H.; Yan, Y.; Sun, J. H-NS Mutation-Mediated CRISPR-Cas activation inhibits phage release and toxin production of Escherichia coli Stx2 phage lysogen. Front. Microbiol. 2017, 8, 652.
- Ershova, A.; Rusinov, I.; Spirin, S.; Karyagina, A.; Alexeevski, A. Role of restriction-modification systems in prokaryotic evolution and ecology. Biochemistry 2015, 80, 1373–1386.
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune strategy. Annu. Rev. Vir. 2020, 7, 371–384.
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Characterization of lytic bacteriophages infecting multidrug-resistant shiga toxigenic atypical Escherichia coli O177 strains isolated from cattle feces. Front. Public Health 2019, 7, 355.
- Tang, S.; Orsi, R.H.; Luo, H.; Ge, C.; Zhang, G.; Baker, R.C.; Stevenson, A.; Wiedmann, M. Assessment and comparison of molecular subtyping and characterization methods for Salmonella. Front. Microbiol. 2019, 10, 1591.
- Lee, C.; Choi, I.Y.; Park, D.H.; Park, M.-K. Isolation and characterization of a novel Escherichia coli O157: H7-specific phage as a biocontrol agent. J. Environ. Health Sci. Eng. 2020, 18, 189–199.
- Tanji, Y.; Shimada, T.; Fukudomi, H.; Miyanaga, K.; Nakai, Y.; Unno, H. Therapeutic use of phage cocktail for controlling Escherichia coli O157: H7 in gastrointestinal tract of mice. J. Biosci. Bioeng. 2005, 100, 280–287.
- Sheng, H.; Knecht, H.J.; Kudva, I.T.; Hovde, C.J. Application of bacteriophages to control intestinal Escherichia coli O157: H7 levels in ruminants. Appl. Environ. Microbiol. 2006, 72, 5359–5366.
- Stanford, K.; McAllister, T.; Niu, Y.; Stephens, T.; Mazzocco, A.; Waddell, T.; Johnson, R. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157: H7 in feedlot cattle. J. Food Protect. 2010, 73, 1304–1312.
