The efficiency of (chemo-)radiotherapy for rectal cancer is not only determined by the impact on the tumor cells themselves, but also by the highly individual surrounding tumor microenvironment, including immune cells. However, many aspects of the radiation-induced immune response remain to be fully understood. This review summarizes existing literature about the effects of (chemo-)radiotherapy on the rectal cancer immune microenvironment, which can be both tumor- suppressive or pro-tumorigenic, by either promoting an effective anti-tumor immune response or mediating resistance. We further aim to highlight potential immune-modulating combination therapies, such as immune checkpoint inhibitors, that offer individualized approaches to target the heterogeneous tumor immune microenvironment.
- rectal cancer
- radiotherapy
- tumor immune microenvironment
1. Introduction
Colorectal cancer (CRC) represents the third most diagnosed cancer and the second leading cause of cancer mortality for both sexes combined, comprising approximately 1 in 10 cancer cases and deaths worldwide in 2020 [1]. While the incidence of CRC for ≥ 50-years-old adults showed a favorable trend in recent years, the incidence recently increased in the group of young adults (<50 years-old) in multiple high-income countries [1–4].
The standard treatment for locally advanced rectal cancer (LARC) involves a multi-modal approach commonly including neoadjuvant chemoradiotherapy (nCRT) or short- course radiotherapy (SCRT), total mesorectal excision and adjuvant chemotherapy (CT) [5]. SCRT generally involves five fractions at a dosage of 5 Gy within one week, followed by early surgery, while conventional nCRT is usually administered in a long-course (LCRT) regimen of 1.8–2 Gy fractions, leading up to a total dosage of 45–50.4 Gy before delayed surgery [6–8]. The recommended chemotherapy agents used within nCRT regimens for rectal cancer are typically capecitabine or 5-fluorouracil (5-FU) [5,9,10]. Although im- provement of local tumor control was described as the main advantage of neoadjuvant radiotherapy (nRT) and nCRT, an increase in overall survival (OS) could not be demon- strated by their administration [8,11,12]. Recently, total neoadjuvant therapy (TNT) has evolved into a promising novel approach for rectal cancer therapy, combining CRT and sys- temic CT prior to surgery [13–15]. As potential benefits of TNT, in comparison to standard CRT for LARC, an increase in pathological complete response (pCR), disease-free survival and OS as well as reduced risk for distant metastasis have been reported [16]. Radiation is a crucial part of combination therapy strategies for rectal cancer and it is imperative to continuously improve its efficiency. Yet, the response to (C)RT still varies widely amongst patients treated for rectal cancer, and the molecular mechanisms underlying this individu- ality as well as potential predictors remain to be fully understood [17,18]. Consequently, additional research is required to investigate the effects of radiation on rectal cancer and its contribution to mechanisms of treatment resistance.
Over the past two decades, the perception of cancer has shifted from a view centering the cancer cell to a concept embedding it in a network of stromal components such as fibroblasts, endothelial cells and a variety of immune cells, summarized as the tumor microenvironment (TME) [19,20]. The diverse TME has been recognized as an important factor in tumor progression and metastasis and represents an attractive target for therapy approaches [21,22]. Additionally, increasing attention has been directed towards elucidating its role in resistance against systemic therapies [23] and RT [18,24].
Colon and rectal cancer, even though being highly related, can be considered distinct diseases [25,26]. Importantly, their difference also reflects in the tumor immune microen- vironment (TIME) [27,28]. An important molecular difference between colon and rectal cancer is the gradient in microsatellite instability, which was detected by Salem et al. in 22.3% of right-sided colon tumors, 7.1% of left-sided colon tumors, but only in 0.7% of rectal cancers [29]. In a study by Lin et al., microsatellite instability-high CRC showed a more favorable response to immunotherapy and enhanced immunogenicity in comparison to microsatellite stable CRC, which was observed to be prone to an inhibitory TME [28]. Additionally, the authors speculated that microsatellite instability-high colon and rectal cancer might exhibit differences in their TME and immunologic features [28]. Consistent with these findings, Mezheyeuski et al. concluded from the comparison of immune cell com- positions in pre-treated tumor samples that the level of natural immune activation seems to be lower in rectal than in colon cancer [27]. Therefore, it is crucial to consider the disparities between the TIME of rectal and colon cancer when conducting cancer therapy research.
In line with the former cancer-cell-centered understanding of malignant tumors, for decades, the impact of RT on the cancer cells’ DNA was the main focus of research, neglect- ing its interconnected effects on the TME, such as inflammation, immunomodulation, and extracellular matrix remodeling [24,30]. However, several previous studies suggested that RT and CRT might have stimulating effects on the rectal cancer TIME, as seen in altered infiltration of lymphocytes and dendritic cells (DCs) [31–36]. Yet, (C)RT might also prompt immunosuppressive changes in the rectal cancer TIME by decreasing the number of B cells [31] or upregulating the programmed cell death ligand 1 (PD-L1) [37,38]. Irradiated rectal cancer cells were also shown to induce immune escape mechanisms to circumvent phagocytosis and immune recognition [39]. Thus, immunological effects of radiation within the TME can lead to resistance and recurrence in multiple manners, making the combination of RT and immunotherapy a promising therapeutical approach [24].
This review focuses on the effects of radiation on the rectal cancer TIME, whilst em- phasizing the distinct roles of immune cells. Nevertheless, considering the current clinical practice, where RT is often combined with CT, we have expanded our literature review to in- clude the effects of CRT. Further, even though their differences are increasingly recognized, colon and rectal cancer are still commonly combined as single entity based on anatomical, functional, and histological similarities [26]. Therefore, since rectal cancer-specific literature on certain topics remains limited, we considered it necessary to occasionally reference results from CRC studies in order to complement findings in the context of rectal cancer or propose potential avenues for further rectal cancer-specific investigation.
2. (Chemo-)Radiotherapy’s Dual Impact on the Rectal Cancer TIME:
Immuno-Activating and -Suppressive Effects and Their Therapeutic Implications
Multiple studies have indicated that nCRT can increase the level of immune activation within the rectal cancer TIME via alteration of immune cell infiltration and effector activity, potentially associated with enhanced anti-cancer responses [31–36]. However, radiation can also trigger processes within the TIME that can lead to cancer progression or relapse, many of which are associated with immunosuppression, mediated by different cell types such as macrophages and MDSCs [40,41]. The divergent effects of radiation on the rectal cancer TIME will be explained in the following sections, beginning with the initial aspects of the immune response, and followed by subsequent categorization based on specific cell types.
2.1 Immune Cell Recruitment, Infiltration, and Cytokine Signaling
Irradiation generally triggers an immunogenic cell death (ICD), a process characterized by the release of antigens, cytokines and adjuvant components, driving adaptive immune responses in immunocompetent hosts [42–45]. The adjuvant components, also termed as damage-associated molecular patterns (DAMPs), represent danger signals required for the recruitment of antigen-presenting cells (APCs) [44,45]. A correlation between the TME and the tendency of cancer cells to undergo immunogenic cell death, and therefore priming of an anti-tumor response, has been described in different cancer entities [45–47]. A critical step for immune surveillance is the relocation of immune cells into the tumor site. The infiltration of innate and adaptive immune cells into the TME is known to create a dynamic network of interactions and to play an important role in regulating tumor progression [48–50]. Radiation has been reported to alter immune cell infiltration in different cancers, including rectal cancer, through three main mechanisms [51]: (i) vascular remodeling [52,53], (ii) increase in adhesion molecules [53,54], and (iii) chemokine induction [55,56].
Upon RT or CRT of rectal cancer, the proliferation of tumor and endothelial cells (ECs) was observed to decrease, whilst the expression of adhesion molecules increased, leading to enhanced infiltration of leukocytes [53]. Interestingly, in human CRC tissue, the number of proliferating ECs was found to correlate inversely with infiltration of leukocytes and was identified as a negative prognostic factor [57]. Furthermore, a decline in tumor microvessel density after radiation could be seen in rectal cancer but not in normal rectal mucosa [53]. Therefore, in addition to its direct cytotoxic effects, radiation may facilitate immune cell invasion and contribute to the activation of a local anti-tumor immune response in rectal cancer via modifications in the tumor and its adjacent vascular bed [53].
Lee et al. evaluated the systemic immune response after nCRT for rectal cancer and reported a decrease in peripheral cytokines associated with tumor progression and resistance, one month after CRT [58]. C-C motif chemokine ligand (CCL)2 and CCL3, which have been associated with CRC progression [59–61], presented a significant decline one month post-CRT [58]. In addition, CCL2 was reported to be involved in the recruitment of immunosuppressive immune cells [61,62]. The C-X-C motif chemokine ligand (CXCL)12, also known as stromal cell-derived factor-1 alpha (SDF-1α), and latency-associated peptide (LAP) levels, used to evaluate transforming growth factor-ß (TGF-ß) levels, showed an initial decrease upon CRT application followed by an increase during the first month after CRT termination and decline afterwards [58]. CXCL12 [63] and TGF-ß [64,65] have both been implicated in processes contributing to an immunosuppressive microenvironment in CRC, including recruitment of immunosuppressive immune cells. In line with these findings, another study comparing nRT-treated (5 5 Gy) and non-irradiated surgically resected rectal cancer samples found lower active TGF-β1 in tumor samples of the irradiated group [66]. However, Yasui et al. observed opposing results: when comparing rectal cancer patients who received nCRT (50.4–66 Gy) to patients who did not, TGF-ß was found upregulated (TGFB3) and immuno-fluorescent staining for TGF-β1 revealed that it was derived by cancer cells following nCRT [67]. Analysis of further marker genes, such as signal transducer and activator of transcription 3 (STAT3) and SRY-box transcription factor 2 (SOX2), revealed induction of an immunosuppressive TIME status [67]. Possible reasons for this discrepancy, that should be further investigated in rectal cancer, are dosage differences as well as the influence of CT in a CRT regimen compared to RT only.
Additionally, Lee et al. [58] observed a decrease in interleukin (IL)-12 serum levels upon CRT, which started to recover one month after termination of CRT. Notably, IL-12 is a known promoter of anti-cancer immunity, as shown in multiple preclinical models for different cancer entities including CRC [68–70]. Moreover, previous research has indicated that IL-12 can potently activate cluster of differentiation (CD)8+ T and natural killer (NK) cells and stimulate interferon (IFN)-γ production [71].
IFNs type I (IFN-α and IFN-β) and type II (IFN-γ) play a key role in the coordination of anti-tumor immunity [72]. In the TME of CRC, IFN-γ is produced by a variety of cell types, with T cells and NK cells being the main sources [73]. As most inflammatory cytokines, IFN-γ exhibits pleiotropic effects, which can be influenced by the diverse TME [74,75]. In CRC, its anti-tumor properties include the upregulation of the major histocompatibility complex (MHC) class II receptor HLA-DR (human leukocyte antigen DR) [76] and apoptosis promotion in stem-like cancer cells [77]. To date, most available studies have primarily focused on IFN modulatory effects on CRC, whilst studies specifically investigating its influence on rectal cancer remain scarce. Nevertheless, pathway analysis of upregulated genes comparing pre- and post-(C)RT rectal cancer samples of good responders, revealed a significant upregulation of IFN-γ response, showing its importance in mediating an efficient anti-tumor immune reaction after RT [78]. Furthermore, immune biomarker scores of the IFN-γ signature [36] and IFN-γ expression measured via immunohistochemistry (IHC) [79] were found increased in LARC patient specimens following nCRT.
Plasmacytoid dendritic cells (pDCs) are a subset of DCs specialized in producing type I IFNs [80,81]. It was observed that pre-CRT rectal cancer contained just few IFN-α- expressing pDCs, whilst this proportion was increased after CRT, leading to the conclusion that CRT could enhance IFN-α expression in rectal cancer [82]. In established mouse models using the poorly immunogenic MC38 colon cancer cell line, it was found that the IFN-α gene transduction could reduce the cancer cell’s tumorigenicity and evoke a potent anti-tumor immune response with increased infiltration of cytotoxic T lymphocytes (CTLs) [83,84]. These findings highlight the importance of specific investigation whether radiation-triggered elevation of IFN-α in rectal cancer could contribute to an effective RT response by enhancing CTL infiltration and function to boost anti-tumor immunity. CRT further induced the expression of CXCL10 and CCL4 in pDCs infiltrating rectal cancer [82], chemokines that have been associated with T-cell migration [85], implying that pDCs might play a role in T cell trafficking to rectal tumors after CRT.
To conclude, RT and CRT have the potential to enhance the crucial initial steps of the anti-tumor immune response: recruitment and infiltration of immune cells. Through danger signal release upon radiation, immune cells are attracted to the tumor site and their infiltration might be facilitated through modifications of the tumor vasculature and cytokine levels. However, it is important to note that literature on cytokine alterations after rectal cancer RT is scarce, highlighting the need for further investigation in this field. On the other hand, it is crucial to remember that radiation is physically detrimental towards cells, including the cells of the TIME. Therefore, the depletion of immune cells collectively must be considered as important process of immunosuppression upon (C)RT [86]. This effect influences the composition of the TIME since lymphocytes are more radiosensitive than other immune cells, such as macrophages or granulocytes [87,88]. Interestingly, among lymphocytes, regulatory T cells (Tregs), which are associated with immune evasion by tumors, appear to be the most radioresistant cell type [89,90], strongly suggesting that immunosuppressive elements are less affected by the process of physical destruction within the TIME.
Therefore, radiotherapy exerts both: immunostimulation and immunosuppression in terms of general immune cell infiltration and differentiation. Therapeutically, these effects could potentially be enhanced or inhibited. (Pre)clinical trials have evaluated the modification of IFN pathways in addition to RT [91–93]. However, the results remain controversial and raise the question of potential toxicity [93].
2.2. Treatment-Induced Effects on Innate Immunity
2.2.1. Tumor-Associated Macrophages
Tumor-associated macrophages (TAMs) are a main component of the TIME and known to play a role in tumor growth, progression, and metastasis [94–96]. Although their infiltration in CRC is initially associated with tumor suppression by orchestrating the anti-tumor immune response in early stages [97], overall it remains controversial which effect they have on the tumor [98,99]. They exhibit remarkable functional diversity as they perform central tasks in phagocytosis and antigen presentation and show a high level of plasticity under immunomodulatory conditions [100,101]. Their ample heterogeneity has been shown to be influenced by the TME in CRC [102]. To account for their diversity, their phenotype has been classified in M1 and M2 macrophages based on their surface markers, secretory profiles, and functions [103–105]. This initial classification mirrored the basic T helper (Th)1 cell and Th2 cell polarization profiling [103]. Whilst the M1 phenotype is characterized as proinflammatory and tumoricidal, the M2 type is associated with repair, tuning of the immune response and tumor progression [104–106]. However, it has become increasingly clear that there are several subsets of TAMs, highlighting their great heterogeneity and calling for redefinition of the traditional classification [107,108]. Additionally, their plasticity, allowing the reprogramming of their phenotype according to environmental settings, is increasingly acknowledged [100,101].
Using flow cytometry after ex vivo irradiation of primary rectal cancer tissue samples, Stary et al. reported that low-dose RT resulted in a change in TAM polarization from the M2-like to the pro-inflammatory M1-like phenotype [109]. Furthermore, the phagocytic ca- pacity of irradiated TAMs was found to be enhanced, going in line with downregulation of programmed cell death receptor 1 (PD-1) [109], a well-known inhibitor of phagocytosis [110]. Additionally, when these findings were translated in vivo by analyzing irradiated patient- derived rectal cancer samples, Stary et al. observed an increased M1/M2 ratio upon RT. The study furthermore presented results indicating that this effect might be mediated by extracel- lular vesicles released from irradiated tumor cells containing factors driving the phenotypic switch, but the precise underlying molecular pathways require further investigation [109].
Gene expression profiling performed by Wilkins et al., comparing pre-(C)RT vs. post- (C)RT rectal cancer samples, showed longitudinal upregulation of CYBB and CD68, genes reflecting increased macrophage populations, in good responders, unlike poor respon- ders [78]. The authors noted that at pre-treatment baseline, poor responders demonstrated an intrinsic inflammatory phenotype with upregulation of CD163, a marker for the M2-like macrophage subtype. It was suggested that a favorable response to (C)RT in rectal cancer is attributed to a transition from an immunologically “cold” to “hot” state [78]. To conclude, under specific conditions and depending on the TME, TAMs might be an important element of the anti-tumor response after rectal cancer RT.
Strongly contrasting these findings, Cho et al. observed that in LARC patient samples after nCRT, TAMs mainly changed their phenotype to M2, suggesting that they suppress the anti-tumor immune reaction [31]. These findings are in line with results by Yasui et al., who showed more M2 macrophage infiltration following nCRT compared to tumors pre- nCRT [67]. Moreover, they observed an elevation in TGF-ß expression by the tumor cells [67], which is known to attract immunosuppressive TAMs [111]. Remarkably, these results contradict the findings by Stary et al., who reported a differentiation of TAMs to the M1 phenotype following irradiation [109]. The main reason for this discrepancy could be differentially applied forms of RT: Stary et al. used material from patients who received SCRT at a dosage of 2 2.5 Gy per day over a course of five days [109]. In the other two studies, however, all applied RT regimens were performed with at least 50 Gy. Further, the neoadjuvant treatment of the latter included chemotherapeutics. Essentially, this discrepancy reflects that RT can elicit opposing effects on the TIME, which may depend on the applied dosage or its fractionation.
Moreover, the classification of TAMs into M1- and M2-polarized macrophages is an insufficient approach to reflect the TIME reality. Some macrophages can express cell markers which occur in both subtypes without allowing distinction, such as CD163 which originally characterizes M2 polarization but can also be found in more M1-like macrophages [112]. This underlines the great heterogeneity of TAM subtypes and their intermediate forms, which can be found in the rectal cancer TME, and therefore contributes to inconsistent findings regarding their function and distribution [113,114]. Pinto et al. evaluated how human-derived macrophages maintained in vitro would react to RT [115]. The macrophages did not only stay viable but also showed a pro-inflammatory polarization: anti-inflammatory gene markers (CD163, MRC1, VCAN) as well as the immunosuppressive cytokine IL-10 were decreased, whilst pro-inflammatory markers (CD80, CD86, HLA-DR) were increased, which could add to anti-cancer immunity. Moreover, the irradiated macrophages exerted pro-invasive and -angiogenic effects when cultured with a colon cancer cell line [115]. Yet, it remains to be tested whether these results can be translated to rectal cancer and to an in vivo situation.
Thymidine phosphorylase (TP) expression by TAMs could have an influence on pro- gression and treatment of rectal cancer. However, its role is not yet fully understood: on the one hand it promotes CRC growth due to pro-angiogenetic and anti-apoptotic effects on the cancer cells [116]; on the other hand, it is responsible for the catalyzation of the widely used chemotherapeutic 5-fluorouracil into its more active nucleoside form, 5-fluoro-2tdeoxyuridine [117]. Interestingly, for rectal cancer patients a significant correla- tion between high TP levels and poor outcome was found after application of nCRT [118]. Additionally, a study found TP production by CD68+ macrophages to be increased after irradiation in samples of rectal cancer patients as well as colon cancer cell lines [119]. Furthermore, monocyte chemoattractant protein-1 (MCP-1/CCL2) was also increasingly expressed by the tumor cells one week after irradiation, indicating a higher recruitment of TAMs into the TME and a cross-talking signaling between cancer cells and TAM recruit- ment [119]. A clinical trial with rectal cancer patients observed a rise in TP levels following RT, although the cell type responsible for its expression was not described [120].
In summary, TAMs can be triggered by radiation to exert pro-tumorigenic effects, mainly through the production of immunosuppressing factors and cytokines, which can affect not only tumor growth and metastasis, but also its treatment outcome (see Figure 1). Nevertheless, further studies using different RT doses and fractionation are necessary to fully understand their roles upon RT, since some evidence suggests positive effects on TAM-mediated anti-cancer immunity. Additionally, the baseline immune status of tumors should be acknowledged when evaluating RT immune effects. Therefore, it is crucial to further evaluate which RT conditions are least likely to activate tumor-promoting TAMs and hinder anti-tumor immunity.
2.2.2. Neutrophils
Next to their role as essential defenders against microbial infections, neutrophils have gained attention as significant regulators in the context of cancer [121,122]. Similar to macrophages, neutrophils can be polarized into tumor-promoting or tumor-suppressing subtypes depending on the TME [123,124]. High neutrophil blood counts were shown to be associated with worse survival of rectal cancer patients [125]. Furthermore, the neutrophil-to-lymphocyte ratio (NLR) has gained attention in recent years as prognostic marker for rectal cancer [126–128], which underlines the important role of neutrophils in anti-tumor immunity. Recently, a meta-analysis revealed that elevated NLR before CRT, but not after CRT, is associated with poorer prognosis [126]. Nonetheless, the prognostic impact of intratumoral neutrophils in rectal cancer remains uncertain [129–132]. Previous studies have indicated that neutrophils play a role in creating an immunosuppressive TME, making them a potential treatment target [122]. However, opposing results have been reported concerning the response of neutrophils upon RT [122]. 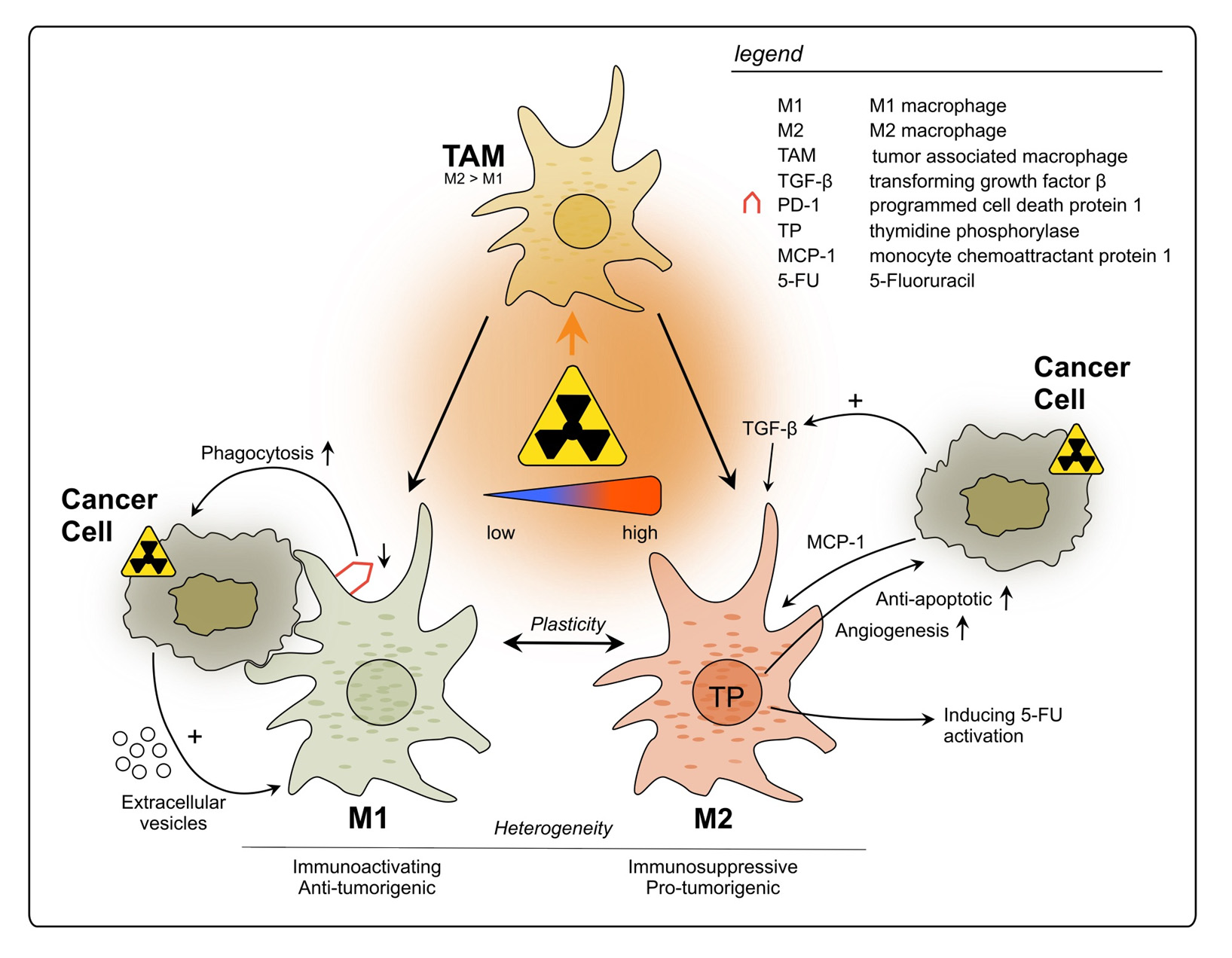
Figure 1. Impact of radiotherapy on tumor-associated macrophages in rectal cancer. TAMs represent a heterogeneous population and exhibit functional diversity. They are classified into two major phenotypes: M1, which is characterized as immune-activating, anti-tumorigenic and M2 with immunosuppressive, pro-tumorigenic properties. Due to high plasticity, TAMs can alter their phenotype depending on the surrounding conditions. Conflicting results have been reported regarding the polarization of TAMs upon rectal cancer RT, with studies describing increase in polarization towards either M1 or M2. This discrepancy may be attributed to differences in the dosage of radiation applied during the treatment.
During the first two weeks of CRT, a decrease in neutrophils in rectal cancer patients’ blood samples was reported, but numbers increased again one month after termination of CRT [58]. Additionally, it was observed that rectal cancer patients who achieved a pCR demonstrated significantly lower peripheral blood neutrophil counts compared to those who did not achieve pCR two weeks after CRT initiation, but not upon the begin- ning of the treatment [58]. Another study found a significant decrease in the intratu- moral and peritumoral numbers of neutrophils five days after RT of rectal cancer [133]. These limited findings suggest that RT may potentially decrease the immunosuppres- sive and tumor-promoting properties of neutrophils in rectal cancer by reducing their numbers. However, studies in mouse models of other tumor entities reported a peak of neutrophil levels after 24 h with a subsequent decline, highlighting their role as initial innate responders [134,135]. Those studies observed that RT could enhance cytokine release leading to increased neutrophil infiltration and to promote a switch towards an anti-tumor phenotype upon RT [134,135]. Reactive oxygen species were found to play a central role me- diating the radiosensitizing, tumor-suppressing activity of irradiated neutrophils [134,135]. Therefore, research should be conducted in rectal cancer after shorter time intervals since neutrophil levels undergo dynamic changes.
However, some studies suggest an unfavorable influence in rectal cancer [136,137]. In a retrospective analysis performed with 73 LARC patients, circulating neutrophils remained stable in numbers during CRT [136]. Furthermore, a high neutrophil blood count post- nCRT correlated with an unfavorable outcome, which was associated with their suppressive effects on T cells [136]. This effect is mediated through the production of NOS and/or the release of arginase I upon their active degranulation or death [138–140].
On the contrary, in another study where the granulocyte blood count of LARC patients was evaluated after nCRT, a significantly lower number of these cells was found when compared to patients who did not undergo preoperative therapy [86]. This could be shown until two days after surgery 4–6 weeks after the nCRT and was interpreted as a sign of immune dysfunction and associated with postoperative complications. However, the granulocyte counts strongly increased after the surgery in both groups, probably related to the well described systemic inflammatory response post-surgical interventions [86].
Yang et al. evaluated in their study whether the NLR could be associated with the risk for distant metastasis in LARC patients [137]. They found not only that a high NLR was indicative for distant metastasis and a poorer survival but also that the NLR rise depended on the used RT modalities, an effect which could be attributed to the exposure of the bone marrow to low-dose radiation during intensity-modulated RT leading to leukocytopenia and therefore triggering a NLR rise. Furthermore, LCRT is believed to lead to a higher NLR than SCRT [137].
Summing up, the effects of rectal cancer RT on neutrophils are controversial and their clinical implications are still poorly evaluated, even though their prognostic relevance is well known. The limited availability of literature makes it currently impossible to provide a definitive assessment of the functional characterization of neutrophils upon rectal cancer (C)RT. Well-defined experimental setups and clinical studies will be crucial the development of treatment modalities that influence neutrophil phenotypes in a manner that favors patients’ outcome.
2.2.3. Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs), firstly described as “natural suppressor cells” in 1978 [141], encompass a heterogeneous group of immature myeloid cells that play an important immunosuppressing role in the TME [142,143]. They can be divided in two main cat- egories: polymorphonuclear (PMN-MDSCs) and monocytic (M-MDSCs) [144–146]. However, due to broad ambiguity regarding clearer definitions of this cell population, the general term MDSC is still widely used [147]. In cancer, they generally exert immunosuppressive ef- fects via multiple direct and indirect mechanisms, including suppression of T cell activation, Treg development and modulation of cytokine production by macrophages [142,143,148]. Accordingly, in rectal cancer MDSCs are primarily known to contribute to immunosuppres- sion and treatment resistance [149,150].
In a LARC cohort of 25 patients, pre- and post-nCRT intratumoral MDSC levels were found significantly higher in non-responders, implying that higher intratumoral MDSCs before nCRT of rectal cancer correlate with non-responsiveness [149]. In line with this finding, high MDSC infiltration in surgical LARC specimens post-nCRT was associated with worst prognosis [150]. Teng et al. found no change in the expression of MDSCs due to CRT in samples of rectal cancer patients, but they observed that those patients who presented lower levels of MDSCs prior to therapy had a better outcome than those showing higher MDSC numbers [34]. Acknowledging the impact of the high individuality of the TIME, measuring MDSC levels could be used to implement personalized treatment strategies by identifying patients who are likely to benefit from CRT and those with poorer prognosis who may require additional interventions [149,150].
Concerning the exact mechanism involved in treatment resistance, specific literature on rectal cancer is lacking. Blood samples from 41 CRC patients were compared to samples from eight healthy donors and a significant expansion of M-MDSCs was found, which exerted immunosuppressive properties in a mixed leukocyte reaction (MLR) assay where the MDSCs were mixed with DCs as well as T cells [151]. T cell proliferation was evaluated after five days by measurement of 3H-thymidine incorporation and shown to be signifi- cantly reduced compared to a MLR with CD14+/HLA-DRhi monocytes. Moreover, patients who received an anti-cancer treatment (surgery, CT, RT, targeted therapy, or a combination of these methods) showed higher levels of M-MDSCs expressing CD38 when compared to treatment-naïve CRC patients. The authors proposed therefore, that CD38 could be a promising target for immunotherapy. However, there are limitations to this study since all treatments were pooled together as a single group and the exact number of rectal cancer patients within the cohort was not defined (“CRC patients”) [151].
The amino acid L-arginine is of relevance for anti-tumor-immunity: classically acti- vated macrophages can convert it into nitric oxide which is able to enhance tumor reoxy- genation under hypoxic conditions and therefore functions as a radiosensitizer through ROS-mediated DNA damage [152]. Furthermore, T cell proliferation and M1 macrophage activation are stimulated by L-arginine [140]. It has been proposed that one essential mech- anism of MDSC-mediated radioresistance in CRC patients is the depletion of L-arginine via the enzyme arginase-1 [153,154]. Leonard et al. showed that MDSCs exerted these proper- ties in a mouse model using CT26 colon cancer cells in which they impeded activation of anti-tumorigenic macrophages via L-arginine consumption, and thereby observed a radio- protective effect [155]. Furthermore, they observed high counts of arginase-1-producing MDSCs as well as neutrophils in the blood of 235 LARC patients compared to 15 healthy donors. It was not evaluated whether RT would change the number of MDSCs in the patients; however, the authors strongly suggested that MDSCs would impair RT in LARC patients via inhibition of the above-mentioned mechanisms [155].
In a LARC cohort of 25 patients, it was observed that intratumoral MDSCs were effec- tively reduced by nCRT in responders and non-responders; however, the exact mechanism was not specified [149]. Moreover, a subset of immature myeloid cells characterized as HLA-DR−/CD33+/CD16−/CD11b+ was significantly less pronounced in tumors of good responders compared to non-responders. However, blood levels of this subset were strongly increased after 6–8 weeks in responders and non-responders, shortly before surgery. These cells were experimentally found to have a strong suppressive effect on TILs, compared to other myeloid cell subsets [149], probably due to direct contact-mediated inhibition of T cells [156], but not exclusively. Even though not having direct immunosuppressive abilities, by possible colonization of the tumor, circulating MDSC subsets could exert tumor-promoting effects post-treatment.
A trend towards MDSC reduction was also observed in colon cancer mouse models, where single high-dose radiation decreased MDSCs 14 days after irradiation in a mechanism depending on CD8+ cross-priming DCs, IFN-γ secretion and CD40L-expressing CD4+ T cells [157]. Shortly after RT, increased infiltration of CD8+ T cells was found to reverse the immunosuppressive TIME state via IFN-γ production, which was suggested to regulate MDSC infiltration and survival, leading to lasting remission [157]. (C)RT of rectal cancer was shown to induce infiltration of CD8+ and CD4+ T cells [31,32,34], DCs [31] as well as IFN-γ upregulation [36,78,79]. Therefore, these effects could be further investigated as triggers of MDSC elimination in response to rectal cancer radiotherapy.
MDSCs are primarily known to contribute to immunosuppression and treatment resistance in rectal cancer [149,150]. Furthermore, novel treatment approaches should be considered to alleviate their contribution to radioresistance, such as immune population- specific depletion, induction of cell maturation or inhibition of their recruitment to the tumor site, as reviewed elsewhere [158]. However, downregulation of MDSC infiltration could be a possible immune-activating effect of (C)RT in rectal cancer. Further research addressing different treatment regimens will be crucial for the full understanding of this scenario.
2.2.4. Natural Killer Cells
With their potent cytolytic function, NK cells are important players in anti-tumor immunity [159]. When compared to normal tissue, lower NK cell counts were observed in rectal cancer [160]. NK cells were shown to engage in a crosstalk with CD8+ T cells in CRC, enhancing the anti-tumor immune response and supporting prolonged survival [161]. Radiation was reported to promote NK cell activity and migration to the tumor site in different tumor entities, depending on the applied dosage and surrounding TIME [162,163]. In LARC, an increase in the number of NK cells and in the expression of NK cell- associated genes was found after long-course CRT, which correlated with better OS [164]. Furthermore, low NK cell activity was identified as an important factor for the develop- ment of metastasis in stage III rectal cancer after surgery [165]. Yet, in a group of rectal cancer patients, nCRT was reported to impair NK cell activity, thus possibly facilitating metastasis, which represents an additional reason for the combination of CRT with im- munotherapy [165]. This study underlined the heterogeneity of the TIME, leading to highly individual (C)RT responses that make it difficult to predict treatment efficiency. NK cells are influenced by a variety of immune cells, such as TAMs, DCs and Tregs so their activity depends on the TIME composition [162,166]. Furthermore, the impact of radiation on NK cell function in different tumor entities depends on the intensity: whilst low-dose ionizing irradiation tends to activate NK cells, high-dose irradiation is more prone to cause partial impairment of NK cell functions [162,167].
To conclude, further specific research considering different radiation regimens is needed to clarify the role of NK cells in the rectal cancer TIME. Nevertheless, NK cells represent a promising target to enhance anti-tumor immunity and are under investigation as strategic effectors of novel immunotherapeutic approaches, such as chimeric antigen receptor-transduced NK (CAR-NK) cells [168].
2.2.5. Dendritic Cells
DCs represent a system of specialized APCs playing a key role in the initiation and regulation of immune responses [169–171]. Whilst immature DCs primarily capture and process antigens, mature DCs are capable of initiating a potent immune response by priming of T cells, which in turn interact with other immune cells, such as B cells and macrophages [169,171]. Regarding rectal cancer, DC density was reported to increase after CRT, and higher levels of these cells at the pre-treated LARC tumor site were found to be associated with positive response to nCRT [31]. Furthermore, correlations between stromal DCs and CTLs were reported, suggesting a CTLs cross-priming by DCs, enhancing the anti-tumor immune reaction in rectal cancer [172].
6-sulfo LacNAc-expressing monocytes (slanMos) represent a subset of monocytes that produce proinflammatory cytokines, mediate tumor-directed cytotoxicity and can acquire DC functions [173,174]. When investigating the impact of nCRT of rectal cancer on the phenotype of infiltrating slanMos and pDCs, Wagner et al. found an increase in the amount of slanMos secreting nitric oxide synthase (iNOS) or TNF-α and of pDCs locally expressing IFN-α [82]. Additionally, a greater amount of the maturation marker CD83 was observed on pDCs in rectal cancer post-CRT [82].
Therefore, CRT could support anti-tumor immune response not only by increasing the amount of DCs, but also via phenotypical alterations, converting them from an immature status into mature cells, affecting cytokine secretion and cross-activation of other immune cells [82]. Due to the central role of DCs within the interconnected TIME [172,175], these alterations in response to RT have the potential to positively influence multiple processes of anti-tumor activity.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15215124
