You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Chronic kidney disease (CKD), defined as the presence of albuminuria and/or reduction in estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, is considered a growing public health problem. The implementation of novel biomarkers in clinical practice is crucial, since it could allow earlier diagnosis and lead to an improvement in CKD outcomes.
- end-stage kidney disease (ESKD)
- cardiovascular disease
- epidemiology
- CKD
- biomarkers
1. Introduction
A biomarker is defined, by a collaborative working group involved with both the United States National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as “a characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions” [1]. This working group definition was formed on the initiative of the NIH with the aim of accelerating the development and clinical application of reliable biomarkers based on shared definitions. Indeed, an “ideal” biomarker is defined with the presence of some analytic features: (1) it should be measured and readily available in biological samples, such as blood or urine; (2) it should be reproducible, non-invasive, and not expensive [2]. In addition, several clinical features should also be provided to complete the biomarker’s definition; it needs to allow for an early detection of a disease status, while it also needs to have high sensitivity and specificity, i.e., the biomarker needs to differentiate the pathologic status from the normal one and from other clinical conditions, as accurately as possible [3]. The effort made by the NIH–FDA working group is considerable ever since it forecasted and tried to solve the problems of biomarker development, from discovery to clinical application. Indeed, once a biomarker is found to be involved in one or more pathophysiological mechanisms of a disease, it may be introduced into clinical practice to see whether it offers advantages in clinical management and after completion of the validation phase that is considered a crucial step [4]. An important example of the pitfalls of biomarker development is illustrated in the chronic kidney disease (CKD) scenario. CKD is a chronic disease characterized by a poor prognosis, due to the strong association with the development of cardiovascular events, all-cause mortality, and renal events such as renal replacement therapies (RRT, i.e., dialysis or kidney transplantation) [5,6]. The Kidney Disease Improving Global Outcomes Work Group (KDIGO), in 2012, defined CKD with the presence of either decreased kidney function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2) and/or albuminuria, namely, an abnormal amount of protein excretion with urine, for at least three months [7]. The global dimension of the disease is so important that CKD started to be considered a relevant public health problem. Indeed, according to the Global Burden of Kidney Disease, the CKD incidence and prevalence increased by 88.76% and 86.95%, respectively, from 1990 to 2016 [8]. Moreover, the mortality attributed to CKD increased by 41.5% between 1990 and 2017, a percentage that exceeded the mortality due to several neoplasms or cardiovascular (CV) disease [9]. Hence, great effort is advocated toward improving clinical decision-making and reinforcing treatment and prevention of CKD. The KDIGO working group proposed a classification called “CGA” that incorporates the cause (C) of kidney disease, as well as the eGFR (G) and albuminuria (A) levels, to stratify risk in patients with CKD and to better address the importance of the underlying disease.
2. General Classification of Biomarkers
Depending on the intended use, biomarkers are classified as diagnostic, pharmacodynamic/response, monitoring, prognostic, or predictive [19]. We focus, in the present review article, on prognostic and predictive biomarkers, as they represent the most developed biomarkers in CKD patients, while they also incorporate characteristics from other categories of biomarkers. A prognostic biomarker is used to identify the probability of a clinical outcome in patients who are already suffering from the disease of interest [1,20]. Furthermore, prognostic biomarkers measure the association between the disease and clinical outcome in the absence of therapy or with standard therapy that all patients are likely to receive. On the other hand, predictive biomarkers are used to determine whether a patient is likely to benefit from a particular therapy. The clinical benefit could be either a good response to a drug if the biomarker is positive or, alternatively, a lack of benefit from the same drug, which can save a patient from drug toxicity or unnecessary side effects [1].
3. Prognostic Biomarkers in CKD
The importance of prognostic biomarkers in the CKD setting is crucial. Indeed, CKD is a multifactorial disease in which risk factors play different roles in different individuals and in different stages of the disease. It was demonstrated, for example, that the presence of type 2 diabetes leads to the development of CKD in up to 30% of subjects. This means that, in these patients, the deleterious pathogenetic mechanisms of diabetes mellitus are sufficient to damage the kidneys with the onset of typical diabetic glomerulosclerosis. Conversely, it is also possible that, in a portion of diabetic patients, the kidneys are injured by the co-existence of arterial hypertension, which causes different lesions (mainly injuring the kidney vessels), with a completely different prognosis.
3.1. Kidney Biomarkers
The assessment of correct risk stratification, i.e., the allocation of CKD patients to the true risk-of-event categories, always represents a difficult challenge for nephrologists and researchers, due to the large variability in etiology and prognosis of CKD [21,22]. In order to accomplish this aim, a growing number of risk prediction models in CKD patients were developed over the past several years. They show how kidney measures, such as albuminuria (or proteinuria) and eGFR, are strong prognostic biomarkers. Indeed, an eGFR reduction to levels below 60 mL/min/1.73 m2 or even a small increase in albuminuria levels is associated with a significantly increased risk for CV events (CV mortality, coronary heart disease, stroke, heart failure), all-cause mortality, and ESKD (the most advanced stage of CKD that requires referral to renal replacement therapies such as hemodialysis), both in the general population and in patients with an already established CKD (Figure 1) [23,24,25].
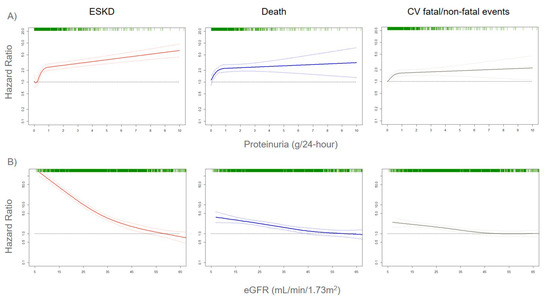
Figure 1. Adjusted risks for end-stage kidney disease (ESKD), death, and cardiovascular (CV) fatal and non-fatal events, by 24-h proteinuria (panel A) or estimated glomerular filtration rate (eGFR) (panel B) levels. Solid lines represent hazard ratios, whereas dashed lines the 95% confidence intervals. Hazard ratios were modeled by means of restricted cubic spline (RCS) due to the non-linear association with the endpoints. Knots are located at the zeroth, 25th, 50th, and 75th percentiles for proteinuria and 15, 30, 45, 60 mL/min/1.73 m2 for eGFR. Risks are adjusted for the four-variable Tangri equation [26]: age, gender, eGFR, and proteinuria. Rug plots on the x-axis at the top (colored green) represent the distribution of observations. Data source: pooled analysis of six cohorts of CKD patients referred to Italian nephrology clinics [27].
Albuminuria and eGFR were also recently used to develop individual risk prediction models for both CV and renal risk in CKD patients. These models employ statistical measures (such as calibration, discrimination, and validation) that represent an essential step before applying risk estimates at the individual level [25,26,28,29]. Moreover, risk prediction models are available for patients with early, moderate, and severe stages of CKD. The addition of albuminuria and eGFR to traditional risk factors included in the model (such as age, gender, presence of diabetes, blood pressure, serum cholesterol) was associated with a significant improvement in risk prediction. Even more importantly, the contribution of albuminuria and eGFR to the prediction of CV events (CV mortality, coronary heart disease, stroke, and heart failure) was found to be greater than traditional CV risk factors [24]. This notwithstanding, a main limitation of available risk score is the insufficient, even absent, consideration of underlying causes of renal disease, which is a crucial point when considering that CKD is a set of multiple etiologies rather than a single disease [12,13]. However, these first prediction models established that measuring albuminuria and eGFR is a central step for assessing risk stratification in CKD patients. Mechanisms of damage of albuminuria and eGFR were partially explained. Albuminuria was shown to exert a direct harmful effect on renal glomeruli and tubules [30]. Moreover, it can also be considered as a systemic marker of endothelial dysfunction, and this explains the reason for which the presence of proteinuria strictly forecasts the onset of CV events in the general population, as well as CKD patients [31]. Similarly, eGFR reduction is linked to an increase of uremic toxins that are responsible for kidney and systemic damage. A drop in eGFR was also associated with the development of coronary atherosclerosis, regardless of the presence of diabetes mellitus, dyslipidemia, previous CV disease, and other comorbidities [32]. Several studies showed that an eGFR decrease is associated with the onset of sudden cardiac death (SCD), with the data being confirmed from the early stage of CKD, i.e., from eGFR < 60 mL/min/1.73 m2 [33,34,35]. For each 10 mL/min decrement in eGFR, SCD risk increased by 11% [33]. SCD accounts for the vast majority of deaths in CKD patients and is also mediated by metabolic and electrophysiological abnormalities [36]. However, both eGFR and albuminuria have limitations in risk prediction. Albuminuria is not specific for any kidney disease, occurring in ischemic, diabetic, and tubulointerstitial nephropathies, as well as in the vast majority of glomerulonephritis and autoimmune diseases [13]. Albuminuria also presents a random variability, since urine protein excretion follows a circadian rhythm that is influenced by posture, exercise, or dietary factors [37]. The eGFR, albeit associated with a poor prognosis, could also be altered by temporary or reversible clinical conditions, such as volume depletions or sub-acute tubulointerstitial diseases [11]. Taken together, proteinuria and eGFR share the limitation of detecting kidney damage often when it is already established and is, thus, not reversible [15]. Furthermore, urine protein excretion is strictly dependent on eGFR levels, which are an expression of the number of functional nephrons in the kidney. In fact, we recently demonstrated that, in a model in which proteinuria is replaced by F-Uprot (proteinuria/eGFR × 100), an expression of the combination of the two biomarkers, the latter allowed refining risk stratification for ESKD outcome in all CKD stages, even in more advanced CKD [27]. Albuminuria levels are strictly dependent and, thus, modified by both systolic and diastolic blood pressure (BP). As long as the eGFR decreases, a given increase in BP is accompanied by an increase in urine protein excretion. This phenomenon was observed in both animal and human studies and is caused by the “remnant nephron effect”, namely, the transmission of systemic hydrostatic pressure to the glomerular microcirculation [38,39]. Moreover, BP is a clinical parameter characterized per se by high variability, which is also detectable during 24-h ambulatory BP measurements (short-term variability) [40]. It was demonstrated that both systolic and diastolic BP variability influence albuminuria levels [41]. Hence, a number of variables were shown to influence albuminuria levels, and this could lead to biased risk estimation, particularly if the risk prediction is based on a single measurement of albuminuria. The ISN indeed highlighted that a consensus needs to be found on how often albuminuria should be measured to warrant a true prediction of cardiovascular and renal endpoints, as well as to monitor the course of CKD [18]. Owing to the evidence that eGFR and albuminuria are able to provide a strong but incomplete prediction of cardiorenal endpoints in CKD patients, the next step of prognostic research focused on the development and assessment of biomarkers that provide useful prognostic information beyond proteinuria and eGFR. A number of markers of inflammation, oxidative stress, or tissue remodeling aroused interest in improving CV and renal risk prediction in CKD patients.
3.2. Markers of Oxidative Stress, Tissue Remodeling, and Metabolism
Myeloperoxidase (MPO) is a biomarker of oxidative stress that fosters nitic oxide consumption and which is associated with the development of atherosclerotic lesions, CV disease, and eGFR decline in CKD [42,43]. A recent observational analysis of the Chronic Renal Insufficiency Cohort (CRIC), which enrolled approximately 4000 patients with CKD in the United States (US), showed that serum MPO levels were associated with the risk of renal outcome, defined as initiation of RRT, 50% eGFR decline, or eGFR ≤ 15 mL/min/1.73 m2 [44]. The key element of this analysis was that the effect of MPO was significant even after adjustment for main confounders, such as baseline eGFR and proteinuria levels. Matrix metalloproteinases (MMPs), endopeptidases involved in tissue development, and homeostasis through the regulation of cell differentiation, apoptosis, and angiogenesis were shown to intervene in inflammatory and fibrotic processes across the kidneys [45,46]. Blood and urine levels of MMPs were linked to renal and CV disease in previous clinical studies in humans. Serum and urine MMP-2, -8, and -9 levels are increased in patients with diabetic CKD, with MMP-9 being significantly associated with the severity of albuminuria [47,48]. Increased plasma levels of TIMP-1 (tissue inhibitor of metalloproteinases-1) predicted the incidence of CKD regardless of inflammatory markers such as C-reactive protein [49]. MMPs and TIMPs also play a role in accelerating the atherosclerotic process by increasing cell migration to the plaque fibrous cap that in turn determines plaque inflammation and rupture [50]. Indeed, the levels of several MMPs (MMP-1, -2, -8, -9) and TIMP-1 were found to be increased in patients with peripheral arterial disease, including those with aneurysms of the arterial wall [51]. Fibroblast growth factor-23 (FGF-23), a hormone involved in phosphorus metabolism that increases progressively as kidney function declines, was significantly associated with mortality, atherosclerotic events, heart failure (HF), and ESKD in CKD patients [52,53].
3.3. Cardiac Biomarkers
Several cardiac biomarkers were investigated, mainly for establishing their role in CV and renal risk prediction in CKD patients. Cardiac troponins (high-sensitivity cardiac troponin (hs-cTnT)) and natriuretic peptides (N-terminal pro-B-type natriuretic peptide (NT-proBNP)) are largely used in CV medicine to diagnose coronary artery disease (CAD) and heart failure (HF), respectively. Both these biomarkers are, thus, an expression of subclinical abnormalities in the heart [54,55]. However, one major problem that makes their introduction in individual risk prediction difficult is that cardiac markers are an expression of both cardiac and kidney dysfunction and cannot discern these two conditions. Natriuretic peptides act by promoting the tubular natriuresis across the kidney and counteracting the effects of renin–angiotensin–aldosterone system, which is triggered by heart failure, as well as renal dysfunction [56]. Concerns about the interpretation of hs-cTnT and natriuretic peptides also derive from the evidence that these marker concentrations are influenced by kidney function levels [56,57]. So far, cardiac markers found more application in the context of prognostic estimation of cardiorenal syndromes (CRS), clinical disorders where an acute or chronic dysfunction of one organ may lead to an acute or chronic dysfunction of the other, thus testifying the strict relationship between the heart and the kidney [58]. In the general population, hs-cTnT and NT-proBNP were shown to be strong predictors for incident HF over time [59,60]. In the setting of CKD, an attempt to evaluate, with appropriate statistical tools, the contribution of cardiac markers to the development of CV events was made using the Atherosclerosis Risk in Communities study (ARIC) population. In this study, examining 7682 non-CKD and 970 patients with CKD stage 1–5, hs-cTnT and NT-proBNP were associated with the development of CV events (defined as the composite of coronary heart disease, stroke, and HF) independently of kidney measures (eGFR and albuminuria) [61]. The finding was confirmed for both CKD and non-CKD patients, as well as for patients with or without previous CV disease. However, the interpretation of these results should be done with caution since the ARIC cohort was stratified, for this analysis, by the presence/absence of CKD, thus limiting the influence of kidney measures on CV risk prediction [61]. Results of the association between cardiac markers and renal outcomes in CKD patients are even more conflicting. The CRIC investigators found, in a cohort of over 3000 CKD patients, that increased plasma levels of growth differentiation factor-15 (GDF-15, a member of the transforming growth factor (TGF)-β cytokine family), hs-cTnT, and NT-proBNP were associated with CKD progression. defined as the onset of ESKD or 50% eGFR decline [62]. However, when all these parameters were added to the prediction model including traditional CV risk factors, the model discrimination (i.e., the ability of the model to separate individuals who develop events from those who do not; see more details in Section 5) did not improve, meaning that their clinical utility was scarce. Moreover, in the Framingham cohort, hs-cTnT was not associated with a faster eGFR decline or with incident CKD [63]. There is, overall, a need for future work to assess the role of cardiac markers in CV and renal risk prediction [61,62,63].
3.4. Filtration and Urinary Biomarkers
Filtration biomarkers and urinary markers were also investigated. The use of cystatin C to estimate GFR (eGFRcys) improved the risk stratification for death, death from CV causes, and ESKD with a large proportion (23%) of patients being reclassified toward true risk estimates when compared with eGFR estimated from serum creatinine (eGFRcrea) [64]. eGFRcys was also shown to predict the onset of SCD in elderly CKD patients [35]. The combination of serum creatinine and cystatin C for estimating eGFR (eGFRcys-crea) allowed clinicians to anticipate the risk prediction of worse outcomes at 85 mL/min, which is well above the 60 mL/min threshold defined by eGFRcrea. β2-microglobulin, another filtration marker, showed a statistical power similar to cystatin C in improving prediction of ESKD, all-cause mortality, and new onset of CV disease beyond eGFRcrea [65]. With respect to urinary markers, some evidence, albeit controversial, was provided for the association of urinary markers of tubule damage (interleukin (IL)-18, kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL)), repair (human cartilage glycoprotein-40 (YKL-40)), and inflammation (monocyte chemoattractant protein-1 (MCP-1)) with the risk of ESKD [66,67]. In fact, when risk prediction models were adjusted for baseline eGFR and albuminuria, the associations of these biomarkers with clinical outcome were consistently attenuated. However, the highest values of KIM-1, MCP-1, and YKL-40 also provided useful risk estimation beyond eGFR and albuminuria in a post hoc analysis of the Systolic Blood Pressure Intervention Trial SPRINT trial [68]. Interestingly, urinary IL-18 and NGAL levels were shown to predict linear eGFR decline over time, an endpoint of growing interest in clinical research [68].
3.5. Prognostic Role of Proteomics, Metabolomics, and Genomics
Proteomics metabolomics and genomics recently provided great input to the implementation of novel biomarkers [69,70,71]. The advantage of these “omics” techniques is to provide a combination of informative peptides/metabolites that are able to classify patients (hence, the appellation of classifiers) into significant clinical or risk categories. A well-depicted classifier in CKD patients is the CKD273, a panel of 273 urine peptides shown to predict, in long-term follow-up cohort studies, eGFR decline with a strong and independent effect to the onset of albuminuria, particularly in diabetic patients [72,73,74]. In further risk prediction models, CKD273 was also able to reclassify about 30% of patients compared with the standard equation that considers eGFR and albuminuria, for the risk of CKD progression [75]. The CRIC investigators described, in a recent manuscript, the association between a panel of 13 urine metabolites and CKD progression [76]. Results of this analysis are encouraging since the levels of four metabolites, namely, 3-hydroxyisobutyrate (3-HIBA), 3-methylcrotonyglycine, citric acid, and aconitic acid, were associated with eGFR decline, with 3-HIBA and aconitic acid levels also significantly associated with the hard endpoint ESKD. Of particular interest is the prognostic role of the genetic causes of CKD. Of all CKD cases diagnosed at a young age (<25 years), an actual 30% are determined by monogenic disorders, and inherited CKD is globally more prevalent (prevalence ranged between 30% and 75%) than previously thought, particularly in the presence of a family history of CKD [77,78]. More importantly, the advent of genome-wide association studies (GWAS) allowed the discovery of several single-nucleotide polymorphisms (SNPs) associated with an increased risk for CKD or with a worse prognosis in patients already affected by CKD [78]. Polymorphisms in the Uromodulin (UMOD) gene region rs4293393, which codifies the most abundant urinary protein in healthy subjects, namely, uromodulin (also called Tamm–Horsfall protein), are associated with an increased risk of incident CKD [79]. A similar role in predicting the onset of CKD was exerted by other SNPs such as Protein Kinase AMP-Activated Non-Catalytic Subunit Gamma 2 (PRKAG2), Longevity Assurance Gene Homologs (LASS2), Disabled Homolog 2 (alias DAB Adaptor Protein 2, DAB2), Dachshund Family Transcription Factor 1 (DACH1), and Stanniocalcin 1 (STC1) [80]. Apolipoprotein L1 (APOL1) gene variants were also studied in CKD patients. APOL1 encodes apolipoprotein L1, which is involved in the lysis of Trypanosoma brucei and other trypanosomes [81]. The G1 and G2 variants of APOL1 were associated with an increased risk of eGFR decline and disease progression to ESKD in CKD populations [82]. Interestingly, information derived from the SNPs were recently combined into a genetic risk score [78]. This score was shown to be associated with eGFR decline and kidney outcome regardless of albuminuria and other renal risk factors encompassing diabetes, history of CV disease, and hypertension. A number of studies assessing the associations between SNPs and kidney measures were carried-out by the United Kingdom (UK) biobank, a large cohort of over 500,000 participants enrolled in 2006–2010, from which genotypic information was widely collected [83]. Analyses of the UK biobank provided a great contribution to the prognostic research in CKD. For example, genetically predicted testosterone and fasting insulin, with the latter being an expression of insulin resistance, were found to be associated with CKD and worse kidney function in men, thus highlighting the possible reasons for discrepancy in CKD prevalence and CKD progression among men and women [84,85]. Intriguingly, a genome-wide association study of UK biobank showed that albumin-to-creatinine ratio (ACR) is dependent on multiple pathways and that an ACR genetic risk score may improve the prediction of hypertension and stroke [86].
4. Predictive Biomarkers in CKD
Predictive biomarkers are used in disparate fields of medicine to assess the likelihood of response to treatments and the individual pathophysiology of the disease. One major example of this strategy is represented by the large use of predictive biomarkers in oncology. Causative mutations of the breast cancer genes 1 and 2 (BRCA1/2) were found to be predictive biomarkers for identifying the response to poly(ADP-ribose) polymerase (PARP) inhibitors [87]. Such a discovery is crucial as BRCA1/2 provide information on the best drug for the individual patient in order to improve their prognosis. While, in oncology, a set of pathophysiological mechanisms is crucial for tumor development, what complicates the application of predictive biomarkers in chronic diseases is that different mechanisms are active in different stages of the disease itself and in different patients [88]. This means that, if a treatment is started on the basis of a blood/urine biomarker level, the individual prognosis may remain unchanged or even worsen, due to the presence of other active mechanisms of damage, as well as, most importantly, different disease entities that cause the chronic decline of renal function through diverse pathophysiological pathways. Notwithstanding, in chronic disease, great research effort was also started with the aim of personalizing treatments following the methodological concept of “the right drug for the right patient”. Hence, the implementation of predictive biomarkers represents a topic of increasing importance.
4.1. Kidney Biomarkers
In nephrology, the most used predictive biomarkers are eGFR and albuminuria. Both these biomarkers can be considered as “dynamic” predictive biomarkers. In fact, their levels change over time with the effects of treatment, such that they can be efficiently used for monitoring the course of CKD and the appropriateness of the therapy followed by the patient. In the past few decades, several interventional studies were carried out testing the effect of nephroprotective drugs on hard endpoints such as mortality, CV events, and ESKD in patients with CKD [89,90,91,92,93,94,95,96]. Although interventions differed between studies, with principally antihypertensive drugs and albuminuria-lowering agents being tested, all these trials pointed out that the CV, mortality, and ESKD risk reductions were strictly associated with a reduction in albuminuria after the start of treatment. Moreover, the magnitude of treatment effect was greater in patients with higher albuminuria levels at the time of the initial visit [95,96]. These findings are reinforced by the evidence that albuminuria changes also played a potentially beneficial role in negative clinical trials. In the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE), which failed in demonstrating the advantage of adding Aliskiren to an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin-receptor blocker (ARB) on CV and renal outcomes, patients who showed an albuminuria reduction in the Aliskiren arm (37%) were largely protected against CKD progression compared with those who did not show a reduction in albuminuria levels [97]. All these pieces of evidence testified that albuminuria has great predictive and prognostic power in CKD patients and, although further studies are needed to find the correct threshold of albuminuria reduction that can confer CV and renal risk protection after an appropriate treatment, there is a general consensus that a 30% reduction in its levels from baseline to six months could be acceptable [98]. With respect to eGFR, it was demonstrated that a doubling of serum creatinine level, which corresponds approximately to a 57% eGFR decline, was able to predict CKD progression in previous clinical trials in diabetic CKD patients [99]. The importance of that evidence is highlighted by the fact that, in these previous trials, eGFR decline correlated with renal outcomes after exposure to nephroprotective treatments, thus affirming its role as a predictive biomarker, in addition to a prognostic biomarker [100]. Since then, the association of lesser eGFR declines with CKD outcomes was tested. A post hoc analysis of the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) clinical trials, two studies that evaluated the efficacy of ARB treatment in patients with diabetes mellitus and nephropathy, showed that 30% and 40% eGFR declines may improve the power of clinical trials if the drug investigated does not determine an acute (within three months of the start of treatment) drop in eGFR [101]. A larger meta-analysis of 37 clinical trials in CKD patients documented a strong association between 30% and 40% eGFR decline in the first 12 months of treatment and the onset of kidney disease progression [100].
4.2. Biomarkers of Tissue Remodeling
In addition to proteinuria and eGFR, other promising predictive biomarkers in CKD were described. A change in serum levels of MMPs after exposure to BB-1101, a synthetic hydroxamic acid-based inhibitor of MMP, was associated with a reduction in proteinuria in experimental models of glomerular damage [102]. A similar effect was observed in diabetic CKD patients who underwent treatment with doxycycline, an antibiotic from the tetracycline family, and who were already treated with renin–angiotensin–aldosterone inhibitors (RAAS-i) [103]. Moreover, MMPs are also involved in the mechanism that leads to CV risk reduction exerted by sodium–glucose cotransporter 2 inhibitors (SGLT2-i) through the activation of RECK (reversion-inducing cysteine-rich protein with kazal motifs), an endogenous inhibitor of MMPs [104]. That mechanism appeared to be independent of proteinuria levels and could also be useful for selecting high-risk normoalbuminuric CKD patients to be enrolled in future clinical trials, who represent a non-trivial proportion of the CKD cohort [21].
4.3. Ultrasound Biomarkers
Evidence is also emerging for a possible role of the renal resistive index (RRI) as a dynamic predictive biomarker. RRI is a Doppler ultrasonographic index, whose increase reflects both renal and systemic vascular impairment [105]. RRI was also found to predict the onset of CV and kidney outcomes in patients with CKD or essential hypertension [106,107]. RRI values are changed over time by different drug classes, such as RAAS-i and SGLT2-i; novel studies will hopefully reveal in the future if these treatment-induced modifications could also predict hard CV and renal endpoints [108,109].
4.4. Predictive Role of Proteomics, Metabolomics, and Genomics
A polymorphism of the angiotensin-converting enzyme gene caused by an insertion/deletion (ACE/ID) modifies the systemic and renal activity of the RAAS, which was recognized to be a trigger of kidney damage [110]. The ACE/DD–ACE/ID polymorphism was able to predict the response to losartan in type 2 diabetic patients enrolled in the RENAAL trial, that is, patients with worse prognosis (D allele carriers) had the best response to losartan [111]. Complex biomarkers and classifiers have a predictive role, in addition to a prognostic role. A set of 21 serum metabolites were selected from a larger panel through a penalized regression analysis, and they were shown to correctly predict the albuminuria response to ARB treatment in type 2 diabetic patients [112]. This classifier revealed that the enzyme nitric oxide synthase 3 (NOS3) is crucial to forecast the response to ARB therapy in diabetic CKD, since it is involved in the molecular mechanism of action of these drugs. A proteomic predictive classifier was developed from the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) study, using plasma proteomics profiles of fibrosis and kidney damage that allowed predicting the albuminuria change in patients treated with RAAS-i [113].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21165846
This entry is offline, you can click here to edit this entry!
