The shift from the terrestrial to the marine environment to discover natural products has given rise to novel bioactive compounds, some of which have been approved for human medicine. However, the ocean, which makes up nearly three-quarters of the Earth’s surface, contains macro- and microorganisms whose natural products are yet to be explored. Among these underexplored marine organisms are macroalgae and their symbiotic microbes, such as Bacillota, a phylum of mostly Gram-positive bacteria previously known as Firmicutes. Macroalgae-associated Bacillota often produce chemical compounds that protect them and their hosts from competitive and harmful rivals.
- seaweeds
- macroalgae
- Firmicutes
- Bacillota
- natural products
1. Introduction
2. Aquatic Bacillota
3. Marine Macroalgae, a Good Source of Bioactive Bacillota
4. Secondary Metabolites of Marine Macroalgae Bacillota and Their Biosynthetic Gene Clusters
| Algae Species | Growth Medium | Bacterial Species | Biosynthetic Gene Cluster | Extract/ Compounds |
Pharmacological Properties | MIC (µg/mL) | References |
|---|---|---|---|---|---|---|---|
| Brown Algae Bacillota | |||||||
| Sargassum wightii | a ZMA * b NA a NA a ZMA |
Bacillus species Bacillus atrophaeus MW821482 |
pks pks nrps Siderophore |
Ethyl acetate extract Ethyl acetate extract |
Antibacterial Antioxidant Antihypertensive Antihypercholesterolamic Anti-inflammatory Anti-hyperglycemic Cytotoxicity Antioxidant Antibacterial Anti-inflammatory Anti-hyperglycemic Antihypertensive Antioxidant Anti-hypercholesterolemic Antibacterial |
6.25–12.5 ⁑ (133–492.04) ⁑ (498.12–735.42) ⁑ (10.21–24.32) ⁑ (5.22–735.45) ⁑ (92.02–759.24) ♯ 29.5 ♯ (133–4167) 6.25–12.5 ⁑ (9.74–788.8) ⁑ (118.1–513.4) ⁑ 713.6 ⁑ (413.2–429.8) ⁑ 22.23 6.25–12.5 |
[83][84][85][86] |
| Anthophycus longifolius | a NA ** NA SWA ZMA * NA MA * NA |
Bacillus subtilis MTCC 10403 | pks pks pks |
(1) (35–38) (2) |
Antibacterial Antibacterial Antibacterial |
3.12–50 3.12–25 ND |
[87][88][89] |
| Sargassum myriocystum | MA * NA |
Bacillus subtilis MTCC 10407 | pks | (26 and 27) | Antibacterial | ND | [90] |
| Fucus serratus | a TSA DSTA MA NA * CB |
Bacillus licheniformis | ND | YbdN protein | Antibacterial | ND | [80] |
| Endarachne binghamiae | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 188.1–209.7 | [91] |
| Sargassum muticum | MA MB |
Bacillus sp. | ND | Acetone extract | Cytotoxicity Antibacterial |
♯ 5.5 174 |
[91] |
| Egregia menziesii | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 203.0–212.3 | [91] |
| Padina gymnospora | a NA ** NA SWA ZMA * NA |
Bacillus amyloliquefaciens | pks | (28–31) | Antibacterial | ND | [92] |
| Zonaria tournefortii | d LB | Bacillus amyloliquefaciens S13 | ND | Volatile compounds | Antimicrobial | 64–>500 | [82] |
| Red algae Bacillota | |||||||
| Hypnea valentiae | a ZMA * MBSA |
Bacillus amyloliquefaciens MB6 (MTCC 12716) | pks pks-nrps ND |
(3–5) and (6–8) (39–41) Ethyl acetate extract |
Antibacterial Antibacterial Antibacterial Anti-inflammatory Anti-hypercholesterolemic Antidiabetic Antioxidant Antibacterial |
0.38–5.00 ¶ (−9.06)–(−10.13) ¶ (−11.33)–(−13.61) 0.78–3.12 3.125–12.5 ⁑ (6.06–675.36) ⁑ 17.30 ⁑ (84.00–639.54) ⁑ (136.78–278.19) 6.25–12.50 |
[93][94][95][96][97][98] |
| Kappaphycus alvarezii | a ZMA * MBSA |
Bacillus amyloliquefaciens MTCC 12713 | pks pks |
(9–12) (22–25) |
Antibacterial Antibacterial |
‡ 2–9 × 10−3 1.56–6.25 ¶ (−9.06)–(−12.61) |
[99][100] |
| Laurencia papillosa | a NA c ZMA * NA a ZMA a NA * NA a NA ** NA SWA ZMA * NA |
Bacillus velezensis MBTDLP1 MTCC 13048 Bacillus velezensis MBTDLP1 Bacillus amyloliquefaciens |
pks ND pks |
(34) Ethyl acetate extract (32 and 33) |
Antibacterial Antibacterial Anti-inflammatory Cytotoxicity Antidiabetic Antioxidant Antibacterial |
0.38 7.5–15 ♯ 17 ♯ (32.3–200) ♯ (120–420) ♯ (107–4127) ND |
[101][102][103] |
| Laurencia pacifica | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 288.1 | [91] |
| Centroceras clavulatum | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 217.1 | [91] |
| Schizymenia dubyi | MB | Bacillus sp. PP19-H3 | pks | (13–21) | Antibacterial | 10–>100 | [71] |
| Green Algae Bacillota | |||||||
| Codium fragile | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 196 | [91] |
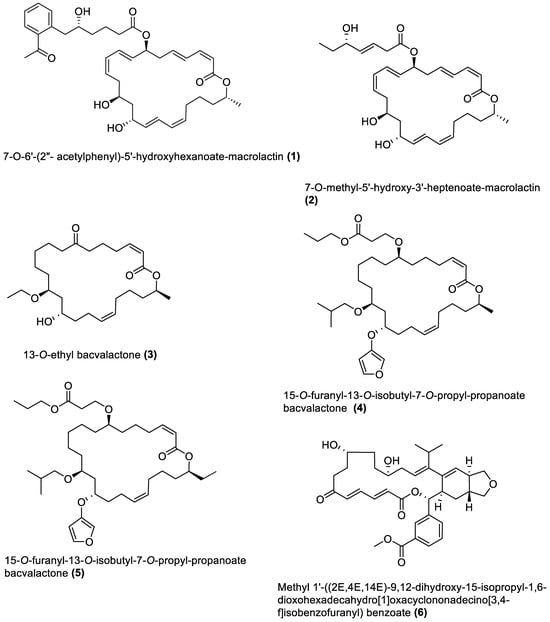
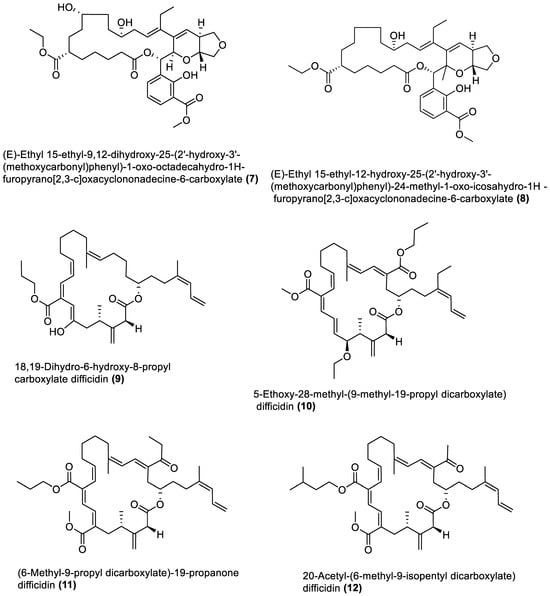
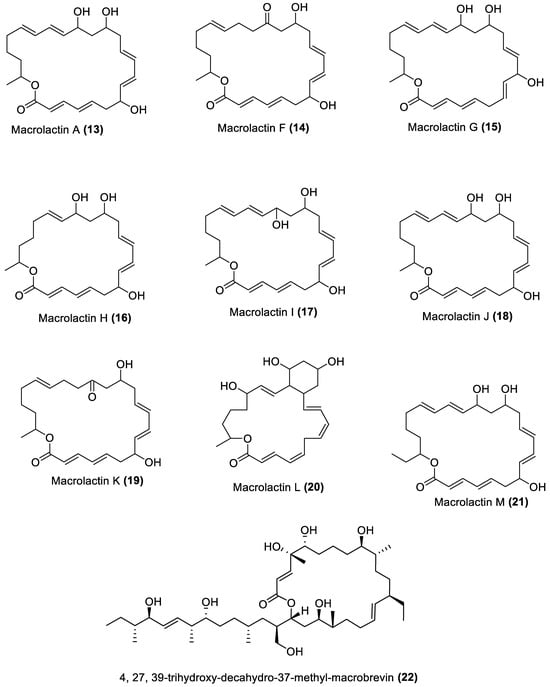
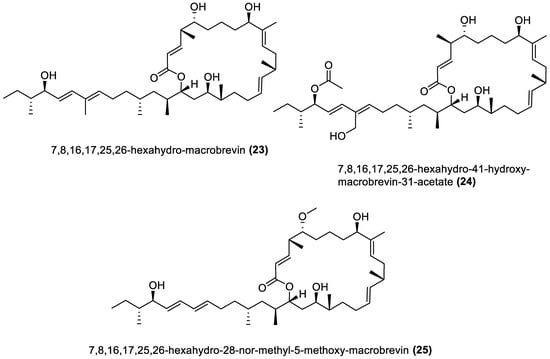
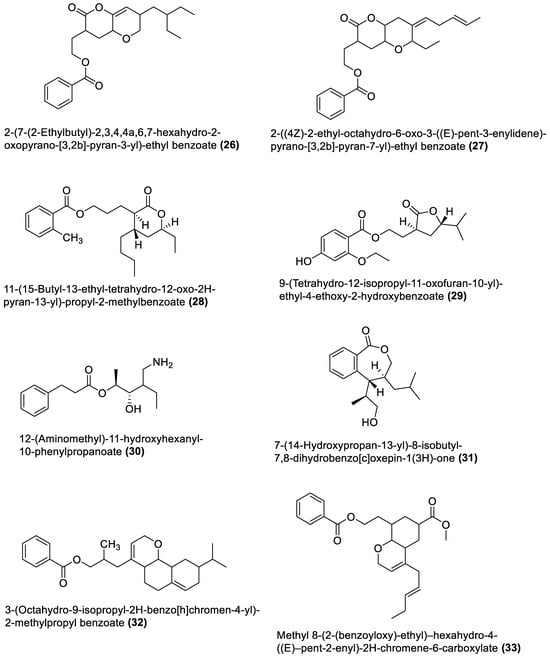
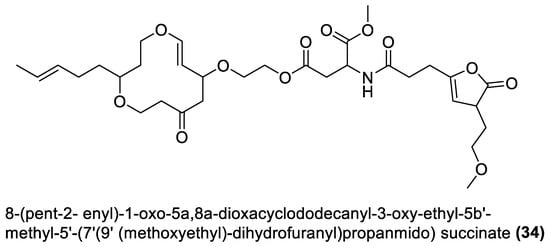
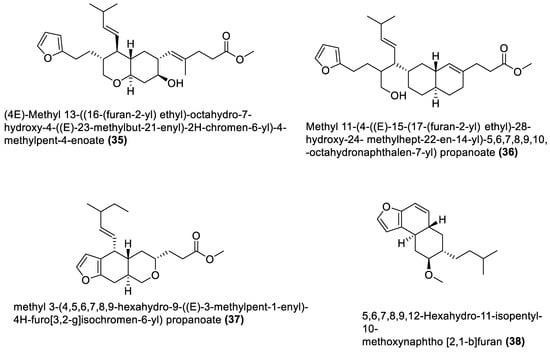
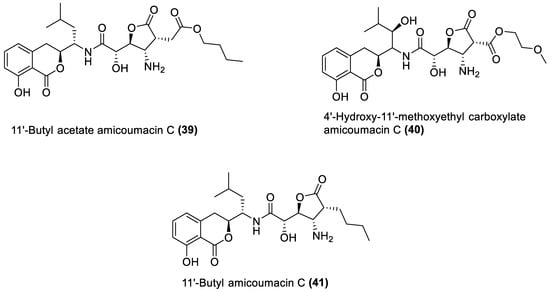
4.1. Macrolides
4.2. Esters
4.3. Furanoterpenoids
4.4. Amicoumacin C Derivatives
5. Pharmacological Properties of the Secondary Metabolites of Marine Macroalgae Bacillota
5.1. Antibacterial Property of Marine Macroalgae Bacillota
5.2. Other Pharmacological Properties of Marine Algae Bacillota
This entry is adapted from the peer-reviewed paper 10.3390/md21110569
References
- Amer, N.F.; Luzzatto Knaan, T. Natural Products of Marine Origin for the Treatment of Colorectal and Pancreatic Cancers: Mechanisms and Potential. Int. J. Mol. Sci. 2022, 23, 8048.
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs 2020, 18, 142.
- Zhou, Q.; Hotta, K.; Deng, Y.; Yuan, R.; Quan, S.; Chen, X. Advances in Biosynthesis of Natural Products from Marine Microorganisms. Microorganisms 2021, 9, 2551.
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488.
- Luzzatto-Knaan, T.; Garg, N.; Wang, M.; Glukhov, E.; Peng, Y.; Ackermann, G.; Amir, A.; Duggan, B.M.; Ryazanov, S.; Gerwick, L.; et al. Digitizing Mass Spectrometry Data to Explore the Chemical Diversity and Distribution of Marine Cyanobacteria and Algae. eLife 2017, 6, e24214.
- Paulus, C.; Rebets, Y.; Tokovenko, B.; Nadmid, S.; Terekhova, L.P.; Myronovskyi, M.; Zotchev, S.B.; Rückert, C.; Braig, S.; Zahler, S.; et al. New Natural Products Identified by Combined Genomics-Metabolomics Profiling of Marine Streptomyces sp. MP131-18. Sci. Rep. 2017, 7, 42382.
- Altmann, K.H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia 2017, 71, 646–651.
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14.
- Liu, Q.A.; Zheng, J.J.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. The Chemistry and Bioactivity of Macrolides from Marine Microorganisms. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 44, pp. 353–401. ISBN 9780444634603.
- Giddings, L.; Newman, D.J. Bioactive Compounds from Marine Extremophiles; SpringerBriefs in Microbiology; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-14360-6.
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528.
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449.
- Bibi, F.; Naseer, M.I.; Azhar, E.I. Assessing the Diversity of Bacterial Communities from Marine Sponges and Their Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 2747–2754.
- Pratheepa, V.; Vasconcelos, V. Microbial Diversity Associated with Tetrodotoxin Production in Marine Organisms. Environ. Toxicol. Pharmacol. 2013, 36, 1046–1054.
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-Producing Bacteria from the Blue-Ringed Octopus Octopus maculosus. Mar. Biol. 1989, 100, 327–332.
- Andrea, S.; Gary, S.; Donald, S.; Stierle, A.; Strobel, G.; Stierle, D. Taxol and Taxane Production by Taxomyces andreanae, an Endophytic Fungus of Pacific Yew. Science 1993, 260, 214–216.
- Viju, N.; Punitha, S.M.J.; Satheesh, S. Antibiofilm Activity of Symbiotic Bacillus Species Associated with Marine Gastropods. Ann. Microbiol. 2020, 70, 11.
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The Seaweed Holobiont: Understanding Seaweed-Bacteria Interactions. FEMS Microbiol. Rev. 2013, 37, 462–476.
- Lachnit, T.; Blümel, M.; Imhoff, J.F.; Wahl, M. Specific Epibacterial Communities on Macroalgae: Phylogeny Matters More than Habitat. Aquat. Biol. 2009, 5, 181–186.
- Lachnit, T.; Meske, D.; Wahl, M.; Harder, T.; Schmitz, R. Epibacterial Community Patterns on Marine Macroalgae Are Host-Specific but Temporally Variable. Environ. Microbiol. 2011, 13, 655–665.
- Aires, T.; Serrão, E.A.; Engelen, A.H. Host and Environmental Specificity in Bacterial Communities Associated to Two Highly Invasive Marine Species (Genus Asparagopsis). Front. Microbiol. 2016, 7, 559.
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Review Chemical Interactions between Marine Macroalgae and Bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–300.
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an Important Environment for Bacteria—Phylogenetic Relationships among New Bacterial Species Isolated from Algae. Phycologia 2013, 52, 14–24.
- Li, X.; Gao, X.; Zhang, S.; Jiang, Z.; Yang, H.; Liu, X.; Jiang, Q.; Zhang, X. Characterization of a Bacillus velezensis with Antibacterial Activity and Inhibitory Effect on Common Aquatic Pathogens. Aquaculture 2020, 523, 735165.
- Armstrong, E.; Yan, L.; Boyd, K.G.; Wright, P.C.; Burgess, J.G. The Symbiotic Role of Marine Microbes on Living Surfaces. Hydrobiologia 2001, 461, 37–40.
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777.
- Egan, S.; Thomas, T.; Kjelleberg, S. Unlocking the Diversity and Biotechnological Potential of Marine Surface Associated Microbial Communities. Curr. Opin. Microbiol. 2008, 11, 219–225.
- Viju, N.; Punitha, S.M.J.; Satheesh, S. An Analysis of Biosynthesis Gene Clusters and Bioactivity of Marine Bacterial Symbionts. Curr. Microbiol. 2021, 78, 2522–2533.
- Dat, T.T.H.; Cuc, N.T.K.; Cuong, P.V.; Smidt, H.; Sipkema, D. Diversity and Antimicrobial Activity of Vietnamese Sponge-Associated Bacteria. Mar. Drugs 2021, 19, 353.
- Kuo, J.; Yang, Y.T.; Lu, M.C.; Wong, T.Y.; Sung, P.J.; Huang, Y. Sen Antimicrobial Activity and Diversity of Bacteria Associated with Taiwanese Marine Sponge Theonella swinhoei. Ann. Microbiol. 2019, 69, 253–265.
- Garg, N.; Luzzatto-Knaan, T.; Melnik, A.V.; Caraballo-Rodríguez, A.M.; Floros, D.J.; Petras, D.; Gregor, R.; Dorrestein, P.C.; Phelan, V.V. Natural Products as Mediators of Disease. Nat. Prod. Rep. 2017, 34, 194–219.
- Ito, T.; Sekizuka, T.; Kishi, N.; Yamashita, A.; Kuroda, M. Conventional Culture Methods with Commercially Available Media Unveil the Presence of Novel Culturable Bacteria. Gut Microbes 2019, 10, 77–91.
- Novik, G.; Savich, V. Beneficial Microbiota. Probiotics and Pharmaceutical Products in Functional Nutrition and Medicine. Microbes Infect. 2020, 22, 8–18.
- Kang, H.; Kim, H.; Yi, H.; Kim, W.; Yoon, J.; Im, W.; Kim, M.K.; Seong, C.N.; Kim, S.B.; Cha, C.; et al. A Report of 43 Unrecorded Bacterial Species within the Phyla Bacteroidetes and Firmicutes Isolated from Various Sources from Korea in 2019. J. Species Res. 2021, 10, 117–133.
- Jemil, N.; Manresa, A.; Rabanal, F.; Ben Ayed, H.; Hmidet, N.; Nasri, M. Structural Characterization and Identification of Cyclic Lipopeptides Produced by Bacillus methylotrophicus DCS1 Strain. J. Chromatogr. B 2017, 1060, 374–386.
- Zheng, C.J.; Lee, S.; Lee, C.H.; Kim, W.G. Macrolactins O-R, Glycosylated 24-Membered Lactones from Bacillus sp. AH159-1. J. Nat. Prod. 2007, 70, 1632–1635.
- Ngashangva, N.; Mukherjee, P.; Sharma, K.C.; Kalita, M.C.; Indira, S. Analysis of Antimicrobial Peptide Metabolome of Bacterial Endophyte Isolated From Traditionally Used Medicinal Plant Millettia pachycarpa Benth. Front. Microbiol. 2021, 12, 656896.
- Hashmi, I.; Bindschedler, S.; Junier, P. Firmicutes. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. ISBN 9780128234143.
- Osei, E.; Kwain, S.; Mawuli, G.T.; Anang, A.K.; Owusu, K.B.A.; Camas, M.; Camas, A.S.; Ohashi, M.; Alexandru-Crivac, C.N.; Deng, H.; et al. Paenidigyamycin A, Potent Antiparasitic Imidazole Alkaloid from the Ghanaian Paenibacillus sp. De2Sh. Mar. Drugs 2019, 17, 9.
- Meene, A.; Herzer, C.; Schlüter, R.; Zayadan, B.; Pukall, R.; Schumann, P.; Schauer, F.; Urich, T.; Mikolasch, A. A Novel Antimicrobial Metabolite Produced by Paenibacillus apiarius Isolated from Brackish Water of Lake Balkhash in Kazakhstan. Microorganisms 2022, 10, 1519.
- Jaruchoktaweechai, C.; Suwanborirux, K.; Tanasupawatt, S.; Kittakoop, P.; Menasveta, P. New Macrolactins from a Marine Bacillus sp. Sc026. J. Nat. Prod. 2000, 63, 984–986.
- Graça, A.P.; Bondoso, J.; Gaspar, H.; Xavier, J.R.; Monteiro, M.C.; de la Cruz, M.; Oves-Costales, D.; Vicente, F.; Lage, O.M. Antimicrobial Activity of Heterotrophic Bacterial Communities from the Marine Sponge Erylus discophorus (Astrophorida, Geodiidae). PLoS ONE 2013, 8, e78992.
- Ou, W.; Yu, G.; Zhang, Y.; Mai, K. Recent Progress in the Understanding of the Gut Microbiota of Marine Fishes. Mar. Life Sci. Technol. 2021, 3, 434–448.
- Schleifer, K.-H. Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes Gibbons and Murray 1978, 5). In Systematic Bacteriology; Springer New York: New York, NY, USA, 2009; Volume 3, pp. 19–1317. ISBN 978-0-387-68489-5.
- Rodrigues, C.J.C.; de Carvalho, C.C.C.R. Cultivating Marine Bacteria under Laboratory Conditions: Overcoming the “Unculturable” Dogma. Front. Bioeng. Biotechnol. 2022, 10, 964589.
- Baltz, R.H. Gifted Microbes for Genome Mining and Natural Product Discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588.
- Baltz, R.H. Genome Mining for Drug Discovery: Progress at the Front End. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab044.
- Van Der Heul, H.U.; Bilyk, B.L.; McDowall, K.J.; Seipke, R.F.; Van Wezel, G.P. Regulation of Antibiotic Production in Actinobacteria: New Perspectives from the Post-Genomic Era. Nat. Prod. Rep. 2018, 35, 575–604.
- Robertsen, H.L.; Musiol-Kroll, E.M. Actinomycete-Derived Polyketides as a Source of Antibiotics and Lead Structures for the Development of New Antimicrobial Drugs. Antibiotics 2019, 8, 157.
- Hallett, M. One Man’s Poison-Clinical Applications of Botulinum Toxin. N. Engl. J. Med. 1999, 341, 118–120.
- Srivastava, S.; Kharbanda, S.; Pal, U.; Shah, V. Applications of Botulinum Toxin in Dentistry: A Comprehensive Review. Natl. J. Maxillofac. Surg. 2015, 6, 152–159.
- Orsini, M.; Leite, M.A.A.; Chung, T.M.; Bocca, W.; de Souza, J.A.; de Souza, O.G.; Moreira, R.P.; Bastos, V.H.; Teixeira, S.; Oliveira, A.B.; et al. Botulinum Neurotoxin Type A in Neurology: Update. Neurol. Int. 2015, 7, 31–35.
- Chen, Y.; Tsai, C.-H.; Bae, T.H.; Huang, C.-Y.; Chen, C.; Kang, Y.-N.; Chiu, W.-K. Effectiveness of Botulinum Toxin Injection on Bruxism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Aesthetic Plast. Surg. 2023, 47, 775–790.
- Hong, S.O. Cosmetic Treatment Using Botulinum Toxin in the Oral and Maxillofacial Area: A Narrative Review of Esthetic Techniques. Toxins 2023, 15, 82.
- Karpiński, T.; Adamczak, A. Anticancer Activity of Bacterial Proteins and Peptides. Pharmaceutics 2018, 10, 54.
- Bandala, C.; Perez-Santos, J.L.M.; Lara-Padilla, E.; Lopez, M.G.D.; Anaya-Ruiz, M. Effect of Botulinum Toxin A on Proliferation and Apoptosis in the T47D Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 891–894.
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288.
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31.
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302.
- Klaenhammer, T.R. Bacteriocins of Lactic Acid Bacteria. Biochimie 1988, 70, 337–349.
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carillo, R. The Generally sRecognised as Safe (GRAS) Process for Industrial Microbial Enzymes. Ind. Biotechnol. 2016, 12, 295–302.
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614.
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus Secreted Polyketides and Peptides Involved in Controlling Human and Plant Pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215.
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-Based Biological Control of Plant Diseases. In Pesticides in the Modern World-Pesticides Use and Management; IntechOpen: London, UK, 2011; Volume 1849, pp. 273–302. ISBN 9789533074597.
- Llario, F.; Falco, S.; Sebastiá-Frasquet, M.T.; Escrivá, J.; Rodilla, M.; Poersch, L.H. The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. J. Mar. Sci. Eng. 2019, 7, 228.
- da Silva, M.A.C.; Cavalett, A.; Spinner, A.; Rosa, D.C.; Jasper, R.B.; Quecine, M.C.; Bonatelli, M.L.; Pizzirani-Kleiner, A.; Corção, G.; de Souza Lima, A.O. Phylogenetic Identification of Marine Bacteria Isolated from Deep-Sea Sediments of the Eastern South Atlantic Ocean. Springerplus 2013, 2, 127.
- Stincone, P.; Brandelli, A. Marine Bacteria as Source of Antimicrobial Compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319.
- Bibi, F.; Yasir, M.; Al-Sofyani, A.; Naseer, M.I.; Azhar, E.I. Antimicrobial Activity of Bacteria from Marine Sponge Suberea mollis and Bioactive Metabolites of Vibrio sp. EA348. Saudi J. Biol. Sci. 2020, 27, 1139–1147.
- Mondol, M.A.M.; Kim, J.H.; Lee, H.-S.; Lee, Y.-J.; Shin, H.J. Macrolactin W, a New Antibacterial Macrolide from a Marine Bacillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 3832–3835.
- Mondol, M.A.M.; Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Cyclic Ether-Containing Macrolactins, Antimicrobial 24-Membered Isomeric Macrolactones from a Marine Bacillus sp. J. Nat. Prod. 2011, 74, 2582–2587.
- Nagao, T.; Adachi, K.; Sakai, M.; Nishijima, M.; Sano, H. Novel Macrolactins as Antibiotic Lactones from a Marine Bacterium. J. Antibiot. 2001, 54, 333–339.
- Mondol, M.A.M.; Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Shin, H.J. New Antimicrobial Compounds from a Marine-Derived Bacillus sp. J. Antibiot. 2013, 66, 89–95.
- Pettit, G.R.; Knight, J.C.; Herald, D.L.; Pettit, R.K.; Hogan, F.; Mukku, V.J.; Hamblin, J.S.; Dodson, M.J.; Chapuis, J.C. Antineoplastic Agents. 570. Isolation and Structure Elucidation of Bacillistatins 1 and 2 from a Marine Bacillus silvestris. J. Nat. Prod. 2009, 72, 366–371.
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and Biodiscovery Hotspots of Macroalgal Marine Natural Products. Nat. Prod. Rep. 2013, 30, 1380–1390.
- Gross, H.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Ballantine, D.L.; Murray, T.F.; Valeriote, F.A.; Gerwick, W.H. Lophocladines, Bioactive Alkaloids from the Red Alga Lophocladia sp. J. Nat. Prod. 2006, 69, 640–644.
- Park, S.J.; Jeon, Y.J. Dieckol from Ecklonia Cava Suppresses the Migration and Invasion of HT1080 Cells by Inhibiting the Focal Adhesion Kinase Pathway Downstream of Rac1-ROS Signaling. Mol. Cells 2012, 33, 141–149.
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567.
- Ren, C.G.; Liu, Z.Y.; Wang, X.L.; Qin, S. The Seaweed Holobiont: From Microecology to Biotechnological Applications. Microb. Biotechnol. 2022, 15, 738–754.
- Kanagasabhapathy, M.; Sasaki, H.; Nagata, S. Phylogenetic Identification of Epibiotic Bacteria Possessing Antimicrobial Activities Isolated from Red Algal Species of Japan. World J. Microbiol. Biotechnol. 2008, 24, 2315–2321.
- Jamal, M.T.; Morris, P.C.; Hansen, R.; Jamieson, D.J.; Burgess, J.G.; Austin, B. Recovery and Characterization of a 30.7-KDa Protein from Bacillus licheniformis Associated with Inhibitory Activity against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococci, and Listeria monocytogenes. Mar. Biotechnol. 2006, 8, 587–592.
- Aleti, G.; Sessitsch, A.; Brader, G. Genome Mining: Prediction of Lipopeptides and Polyketides from Bacillus and Related Firmicutes. Comput. Struct. Biotechnol. J. 2015, 13, 192–203.
- Hamiche, S.; Badis, A.; Jouadi, B.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Identification of Antimicrobial Volatile Compounds Produced by the Marine Bacterium Bacillus amyloliquefaciens Strain S13 Newly Isolated from Brown Alga Zonaria tournefortii. J. Essent. Oil Res. 2019, 31, 203–210.
- Asharaf, S.; Chakraborty, K.; Chakraborty, R.D. Seaweed-Associated Heterotrophic Bacteria: Are They Future Novel Sources of Antimicrobial Agents against Drug-Resistant Pathogens? Arch. Microbiol. 2022, 204, 232.
- Asharaf, S.; Chakraborty, K. Seaweed-Associated Heterotrophic Bacillus altitudinis MTCC13046: A Promising Marine Bacterium for Use against Human Hepatocellular Adenocarcinoma. Arch. Microbiol. 2023, 205, 10.
- Chakraborty, K.; Varghese, C.; Asharaf, S.; Chakraborty, R.D. Antibiotic-Active Heterotrophic Firmicutes Sheltered in Seaweeds: Can They Add New Dimensions to Future Antimicrobial Agents? Arch. Microbiol. 2022, 204, 183.
- Varghese, C.; Chakraborty, K.; Asharaf, S. Pharmacological Potential of Seaweed-Associated Heterotrophic Bacterium Bacillus atrophaeus. Arch. Microbiol. 2023, 205, 6.
- Chakraborty, K.; Thilakan, B.; Kizhakkekalam, V.K. Antibacterial Aryl-Crowned Polyketide from Bacillus subtilis Associated with Seaweed Anthophycus longifolius. J. Appl. Microbiol. 2018, 124, 108–125.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Antimicrobial Polyketide Furanoterpenoids from Seaweed-Associated Heterotrophic Bacterium Bacillus subtilis MTCC 10403. Phytochemistry 2017, 142, 112–125.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide Family of Novel Antibacterial 7-O-Methyl-5′-Hydroxy-3′-Heptenoate-Macrolactin from Seaweed-Associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208.
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-Heterocyclic Derivatives with Antibacterial Properties from Marine Bacterium Bacillus subtilis Associated with Seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583.
- Villarreal-Gómez, L.J.; Soria-Mercado, I.E.; Guerra-Rivas, G.; Ayala-Sánchez, N.E. Antibacterial and Anticancer Activity of Seaweeds and Bacteria Associated with Their Surface. Rev. Biol. Mar. Oceanogr. 2010, 45, 267–275.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Previously Undescribed Antibacterial Polyketides from Heterotrophic Bacillus amyloliquefaciens Associated with Seaweed Padina gymnospora. Appl. Biochem. Biotechnol. 2018, 184, 716–732.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Moving Away from Traditional Antibiotic Treatment: Can Macrocyclic Lactones from Marine Macroalga-Associated Heterotroph Be the Alternatives? Appl. Microbiol. Biotechnol. 2020, 104, 7117–7130.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. A Leap Forward towards Unraveling Newer Anti-Infective Agents from an Unconventional Source: A Draft Genome Sequence Illuminating the Future Promise of Marine Heterotrophic Bacillus sp. Against Drug-Resistant Pathogens. Mar. Biotechnol. 2021, 23, 790–808.
- Kizhakkekalam, V.K.; Chakraborty, K.; Joy, M. Oxygenated Elansolid-Type of Polyketide Spanned Macrolides from a Marine Heterotrophic Bacillus as Prospective Antimicrobial Agents against Multidrug-Resistant Pathogens. Int. J. Antimicrob. Agents 2020, 55, 105892.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Novel Amylomacins from Seaweed-Associated Bacillus amyloliquefaciens as Prospective Antimicrobial Leads Attenuating Resistant Bacteria. World J. Microbiol. Biotechnol. 2021, 37, 200.
- Kizhakkekalam, V.K.; Chakraborty, K. Marine Macroalgae-Associated Heterotrophic Firmicutes and Gamma-Proteobacteria: Prospective Anti-Infective Agents against Multidrug Resistant Pathogens. Arch. Microbiol. 2020, 202, 905–920.
- Kizhakkekalam, V.K.; Chakraborty, K. Pharmacological Properties of Marine Macroalgae-Associated Heterotrophic Bacteria. Arch. Microbiol. 2019, 201, 505–518.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Dhara, S. Difficidin Class of Polyketide Antibiotics from Marine Macroalga-Associated Bacillus as Promising Antibacterial Agents. Appl. Microbiol. Biotechnol. 2021, 105, 6395–6408.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Polyketide-Derived Macrobrevins from Marine Macroalga-Associated Bacillus amyloliquefaciens as Promising Antibacterial Agents against Pathogens Causing Nosocomial Infections. Phytochemistry 2022, 193, 112983.
- Chakraborty, K.; Francis, A.; Chakraborty, R.D.; Asharaf, S.; Kizhakkekalam, V.K.; Paulose, S.K. Marine Macroalga-Associated Heterotrophic Bacillus velezensis: A Novel Antimicrobial Agent with Siderophore Mode of Action against Drug-Resistant Nosocomial Pathogens. Arch. Microbiol. 2021, 203, 5561–5575.
- Francis, A.; Chakraborty, K. Marine Macroalga—Associated Heterotroph Bacillus velezensis as Prospective Therapeutic Agent. Arch. Microbiol. 2021, 203, 1671–1682.
- Chakraborty, K.; Thilakan, B.; Raola, V.K.; Joy, M. Antibacterial Polyketides from Bacillus amyloliquefaciens Associated with Edible Red Seaweed Laurenciae papillosa. Food Chem. 2017, 218, 427–434.
- Gaillard, T.; Dormoi, J.; Madamet, M.; Pradines, B. Macrolides and Associated Antibiotics Based on Similar Mechanism of Action like Lincosamides in Malaria. Malar. J. 2016, 15, 85.
- Das, R.; Rauf, A.; Mitra, S.; Emran, T.B.; Hossain, M.J.; Khan, Z.; Naz, S.; Ahmad, B.; Meyyazhagan, A.; Pushparaj, K.; et al. Therapeutic Potential of Marine Macrolides: An Overview from 1990 to 2022. Chem. Biol. Interact. 2022, 365, 110072.
- Dinos, G.P. The Macrolide Antibiotic Renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983.
- Wang, B.; Wang, L.; Li, Y.; Liu, Y. Heterocyclic Terpenes: Linear Furano- and Pyrroloterpenoids. RSC Adv. 2014, 4, 12216–12234.
- Peterson, L.A. Reactive Metabolites in the Biotransformation of Molecules Containing a Furan Ring. Chem. Res. Toxicol. 2013, 26, 6–25.
- Li, H.; Peng, Y.; Zheng, J. Metabolic Activation and Toxicities of Furanoterpenoids; Elsevier: Amsterdam, The Netherlands, 2016; Volume 10, ISBN 9780128047002.
- Fouche, G.; Nieuwenhuizen, N.; Maharaj, V.; van Rooyen, S.; Harding, N.; Nthambeleni, R.; Jayakumar, J.; Kirstein, F.; Emedi, B.; Meoni, P. Investigation of In Vitro and In Vivo Anti-Asthmatic Properties of Siphonochilus aethiopicus. J. Ethnopharmacol. 2011, 133, 843–849.
- Lategan, C.A.; Campbell, W.E.; Seaman, T.; Smith, P.J. The Bioactivity of Novel Furanoterpenoids Isolated from Siphonochilus aethiopicus. J. Ethnopharmacol. 2009, 121, 92–97.
- Park, H.; Perez, C.; Perry, E.; Crawford, J. Activating and Attenuating the Amicoumacin Antibiotics. Molecules 2016, 21, 824.
- Brinkmann, C.; Kearns, P.; Evans-Illidge, E.; Kurtböke, D. Diversity and Bioactivity of Marine Bacteria Associated with the Sponges Candidaspongia flabellata and Rhopaloeides odorabile from the Great Barrier Reef in Australia. Diversity 2017, 9, 39.
- Ali, A.I.-B.; El Bour, M.; Ktari, L.; Bolhuis, H.; Ahmed, M.; Boudabbous, A.; Stal, L.J. Jania rubens-Associated Bacteria: Molecular Identification and Antimicrobial Activity. J. Appl. Phycol. 2012, 24, 525–534.
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth–Promoting Bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014.
- Albakosh, M.A.; Naidoo, R.K.; Kirby, B.; Bauer, R. Identification of Epiphytic Bacterial Communities Associated with the Brown Alga Splachnidium rugosum. J. Appl. Phycol. 2016, 28, 1891–1901.
- Prieto, M.L.; O’Sullivan, L.; Tan, S.P.; McLoughlin, P.; Hughes, H.; O’Connor, P.M.; Cotter, P.D.; Lawlor, P.G.; Gardiner, G.E. Assessment of the Bacteriocinogenic Potential of Marine Bacteria Reveals Lichenicidin Production by Seaweed-Derived Bacillus spp. Mar. Drugs 2012, 10, 2280–2299.
- Burgess, J.G.; Boyd, K.G.; Armstrong, E.; Jiang, Z.; Yan, L.; Berggren, M.; May, U.; Pisacane, T.; Granmo, Å.; Adams, D.R. The Development of a Marine Natural Product-Based Antifouling Paint. Biofouling 2003, 19, 197–205.
- Thilakan, B.; Chakraborty, K.; Chakraborty, R.D. Antimicrobial Properties of Cultivable Bacteria Associated with Seaweeds in the Gulf of Mannar on the Southeast Coast of India. Can. J. Microbiol. 2016, 62, 668–681.
- Trischman, J.A.; Oeffner, R.E.; De Luna, M.G.; Kazaoka, M. Competitive Induction and Enhancement of Indole and a Diketopiperazine in Marine Bacteria. Mar. Biotechnol. 2004, 6, 215–220.
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J.F. Diversity of Antibiotic-Active Bacteria Associated with the Brown Alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300.
- Penesyan, A.; Marshall-Jones, Z.; Holmstrom, C.; Kjelleberg, S.; Egan, S. Antimicrobial Activity Observed among Cultured Marine Epiphytic Bacteria Reflects Their Potential as a Source of New Drugs. FEMS Microbiol. Ecol. 2009, 69, 113–124.
- Kanagasabhapathy, M.; Sasaki, H.; Haldar, S.; Yamasaki, S.; Nagata, S. Antibacterial Activities of Marine Epibiotic Bacteria Isolated from Brown Algae of Japan. Ann. Microbiol. 2006, 56, 167–173.
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Boudabbous, A.; Stal, L.J.; Cretoiu, M.S.; El Bour, M. Antimicrobial Activities of Bacteria Associated with the Brown Alga Padina pavonica. Front. Microbiol. 2016, 7, 1072.
- Susilowati, R.; Sabdono, A.; Widowati, I. Isolation and Characterisation of Bacteria Associated with Brown Algae Sargassum spp. from Panjang Island and Their Antibacterial Activities. Procedia Environ. Sci. 2015, 23, 240–246.
- Kumar, V.; Rao, D.; Thomas, T.; Kjelleberg, S.; Egan, S. Antidiatom and Antibacterial Activity of Epiphytic Bacteria Isolated from Ulva lactuca in Tropical Waters. World J. Microbiol. Biotechnol. 2011, 27, 1543–1549.
- Quinn, G.A.; Maloy, A.P.; McClean, S.; Carney, B.; Slater, J.W. Lipopeptide Biosurfactants from Paenibacillus polymyxa Inhibit Single and Mixed Species Biofilms. Biofouling 2012, 28, 1151–1166.
- Suresh, M.; Renugadevi, B.; Brammavidhya, S.; Iyapparaj, P.; Anantharaman, P. Antibacterial Activity of Red Pigment Produced by Halolactibacillus alkaliphilus MSRD1—An Isolate from Seaweed. Appl. Biochem. Biotechnol. 2015, 176, 185–195.
- Karthick, P.; Mohanraju, R. Antimicrobial Potential of Epiphytic Bacteria Associated with Seaweeds of Little Andaman, India. Front. Microbiol. 2018, 9, 611.
- Karthick, P.; Mohanraju, R. Antimicrobial Compounds Produced by Lysinibacillus odysseyi Epiphytic Bacteria Associated with Red Algae. Brazilian J. Microbiol. 2020, 51, 1683–1690.
- Horta, A.; Alves, C.; Pinteus, S.; Lopes, C.; Fino, N.; Silva, J.; Ribeiro, J.; Rodrigues, D.; Francisco, J.; Rodrigues, A.; et al. Identification of Asparagopsis armata-associated Bacteria and Characterization of Their Bioactive Potential. MicrobiologyOpen 2019, 8, e00824.
- Vairagkar, U.; Mirza, Y. Antagonistic Activity of Antimicrobial Metabolites Produced from Seaweed-Associated Bacillus amyloliquefaciens MTCC 10456 Against Malassezia spp. Probiotics Antimicrob. Proteins 2021, 13, 1228–1237.
