Chromatin modifications play a crucial role in the regulation of gene expression. The repressor element-1 (RE1) silencing transcription factor (REST), also known as neuron-restrictive silencer factor (NRSF) and X2 box repressor (XBR), was found to regulate gene transcription by binding to chromatin and recruiting chromatin-modifying enzymes. Earlier studies revealed that REST plays an important role in the development and disease of the nervous system, mainly by repressing the transcription of neuron-specific genes. Subsequently, REST was found to be critical in other tissues, such as the heart, pancreas, skin, eye, and vascular. Dysregulation of REST was also found in nervous and non-nervous system cancers. In parallel, multiple strategies to target REST have been developed.
- chromatin modification
- REST/NRSF
- transcriptional regulation
- cancer
1. Introduction
1.1. Gene and Protein Structure
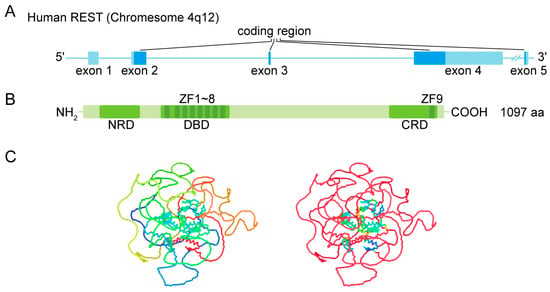
1.2. Nuclear Location
1.3. DNA Binding
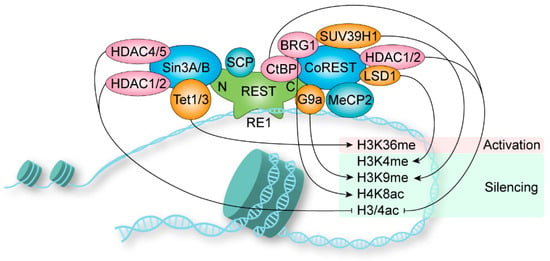
1.4. Coregulator Recruitment
2. REST in Nervous System
2.1. Embryogenesis and Neurogenesis
2.2. Neuronal Differentiation
2.3. Neuronal Survival
2.4. Neuronal Transmission and Synaptic Plasticity
2.5. Pain
2.6. Neuroendocrine
2.7. Intelligence and Memory
2.8. Aging and Alzheimer’s Disease
2.9. Parkinson’s Disease
2.10. Huntington’s Disease
2.11. Epilepsy
2.12. Ischemia
2.13. Psychiatric Disorders
3. REST in Other Systems
3.1. Heart
3.2. Pancreas
3.3. Skin
3.4. Eye
3.5. Vascular
4. REST in Cancer
4.1. Nervous System Cancer
4.2. Non-Nervous System Cancer
5. Conclusion
This entry is adapted from the peer-reviewed paper 10.3390/biom13101477
References
- Chong, J.A.; Tapia-Ramirez, J.; Kim, S.; Toledo-Aral, J.J.; Zheng, Y.; Boutros, M.C.; Altshuller, Y.M.; Frohman, M.A.; Kraner, S.D.; Mandel, G. REST: A mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 1995, 80, 949–957.
- Schoenherr, C.J.; Anderson, D.J. The neuron-restrictive silencer factor (NRSF): A coordinate repressor of multiple neuron-specific genes. Science 1995, 267, 1360–1363.
- Chen, G.L.; Miller, G.M. Alternative REST Splicing Underappreciated. Eneuro 2018, 5, 1–6.
- Shimojo, M.; Lee, J.H.; Hersh, L.B. Role of zinc finger domains of the transcription factor neuron-restrictive silencer factor/repressor element-1 silencing transcription factor in DNA binding and nuclear localization. J. Biol. Chem. 2001, 276, 13121–13126.
- Shimojo, M. Characterization of the nuclear targeting signal of REST/NRSF. Neurosci. Lett. 2006, 398, 161–166.
- Lee, J.H.; Shimojo, M.; Chai, Y.G.; Hersh, L.B. Studies on the interaction of REST4 with the cholinergic repressor element-1/neuron restrictive silencer element. Brain Res. Mol. Brain Res. 2000, 80, 88–98.
- Zhang, Y.; Hu, W.; Shen, J.; Tong, X.; Yang, Z.; Shen, Z.; Lan, W.; Wu, H.; Cao, C. Cysteine 397 plays important roles in the folding of the neuron-restricted silencer factor/RE1-silencing transcription factor. Biochem. Biophys. Res. Commun. 2011, 414, 309–314.
- Yu, H.B.; Johnson, R.; Kunarso, G.; Stanton, L.W. Coassembly of REST and its cofactors at sites of gene repression in embryonic stem cells. Genome Res. 2011, 21, 1284–1293.
- Grimes, J.A.; Nielsen, S.J.; Battaglioli, E.; Miska, E.A.; Speh, J.C.; Berry, D.L.; Atouf, F.; Holdener, B.C.; Mandel, G.; Kouzarides, T. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J. Biol. Chem. 2000, 275, 9461–9467.
- Andres, M.E.; Burger, C.; Peral-Rubio, M.J.; Battaglioli, E.; Anderson, M.E.; Grimes, J.; Dallman, J.; Ballas, N.; Mandel, G. CoREST: A functional corepressor required for regulation of neural-specific gene expression. Proc. Natl. Acad. Sci. USA 1999, 96, 9873–9878.
- Lunyak, V.V.; Burgess, R.; Prefontaine, G.G.; Nelson, C.; Sze, S.H.; Chenoweth, J.; Schwartz, P.; Pevzner, P.A.; Glass, C.; Mandel, G.; et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science 2002, 298, 1747–1752.
- Ballas, N.; Mandel, G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 2005, 15, 500–506.
- Roopra, A.; Qazi, R.; Schoenike, B.; Daley, T.J.; Morrison, J.F. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol. Cell 2004, 14, 727–738.
- Chen, Z.F.; Paquette, A.J.; Anderson, D.J. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat. Genet. 1998, 20, 136–142.
- Wang, Y.C.; Liu, P.; Yue, L.Y.; Huang, F.; Xu, Y.X.; Zhu, C.Q. NRSF deficiency leads to abnormal postnatal development of dentate gyrus and impairment of progenitors in subgranular zone of hippocampus. Hippocampus 2021, 31, 935–9563.
- Jin, S.; Lee, Y.K.; Lim, Y.C.; Zheng, Z.; Lin, X.M.; Ng, D.P.; Holbrook, J.D.; Law, H.Y.; Kwek, K.Y.; Yeo, G.S.; et al. Global DNA hypermethylation in down syndrome placenta. PLoS Genet. 2013, 9, e1003515.
- El Hajj, N.; Dittrich, M.; Bock, J.; Kraus, T.F.; Nanda, I.; Muller, T.; Seidmann, L.; Tralau, T.; Galetzka, D.; Schneider, E.; et al. Epigenetic dysregulation in the developing down syndrome cortex. Epigenetics 2016, 11, 563–578.
- Canzonetta, C.; Mulligan, C.; Deutsch, S.; Ruf, S.; O’Doherty, A.; Lyle, R.; Borel, C.; Lin-Marq, N.; Delom, F.; Groet, J.; et al. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome. Am. J. Hum. Genet. 2008, 83, 388–400.
- Lepagnol-Bestel, A.M.; Zvara, A.; Maussion, G.; Quignon, F.; Ngimbous, B.; Ramoz, N.; Imbeaud, S.; Loe-Mie, Y.; Benihoud, K.; Agier, N.; et al. DYRK1A interacts with the REST/NRSF-SWI/SNF chromatin remodelling complex to deregulate gene clusters involved in the neuronal phenotypic traits of Down syndrome. Hum. Mol. Genet. 2009, 18, 1405–1414.
- Nishihara, S.; Tsuda, L.; Ogura, T. The canonical Wnt pathway directly regulates NRSF/REST expression in chick spinal cord. Biochem. Biophys. Res. Commun. 2003, 311, 55–63.
- Li, H.; Malbon, C.C.; Wang, H.Y. Gene profiling of Frizzled-1 and Frizzled-2 signaling: Expression of G-protein-coupled receptor chimeras in mouse F9 teratocarcinoma embryonal cells. Mol. Pharmacol. 2004, 65, 45–55.
- Johnson, R.; Teh, C.H.; Kunarso, G.; Wong, K.Y.; Srinivasan, G.; Cooper, M.L.; Volta, M.; Chan, S.S.; Lipovich, L.; Pollard, S.M.; et al. REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biol. 2008, 6, e256.
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005, 121, 645–657.
- Singh, S.K.; Kagalwala, M.N.; Parker-Thornburg, J.; Adams, H.; Majumder, S. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature 2008, 453, 223–227.
- Gupta, S.K.; Gressens, P.; Mani, S. NRSF downregulation induces neuronal differentiation in mouse embryonic stem cells. Differentiation 2009, 77, 19–28.
- Thakore-Shah, K.; Koleilat, T.; Jan, M.; John, A.; Pyle, A.D. REST/NRSF Knockdown Alters Survival, Lineage Differentiation and Signaling in Human Embryonic Stem Cells. PLoS ONE 2015, 10, e0145280.
- Satoh, J.; Kawana, N.; Yamamoto, Y. ChIP-Seq Data Mining: Remarkable Differences in NRSF/REST Target Genes between Human ESC and ESC-Derived Neurons. Bioinform. Biol. Insights 2013, 7, 357–368.
- Gao, Z.; Ure, K.; Ding, P.; Nashaat, M.; Yuan, L.; Ma, J.; Hammer, R.E.; Hsieh, J. The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J. Neurosci. 2011, 31, 9772–9786.
- Su, X.; Kameoka, S.; Lentz, S.; Majumder, S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol. Cell. Biol. 2004, 24, 8018–8025.
- Bai, G.; Zhuang, Z.; Liu, A.; Chai, Y.; Hoffman, P.W. The role of the RE1 element in activation of the NR1 promoter during neuronal differentiation. J. Neurochem. 2003, 86, 992–1005.
- Kim, S.M.; Yang, J.W.; Park, M.J.; Lee, J.K.; Kim, S.U.; Lee, Y.S.; Lee, M.A. Regulation of human tyrosine hydroxylase gene by neuron-restrictive silencer factor. Biochem. Biophys. Res. Commun. 2006, 346, 426–435.
- Aoki, H.; Hara, A.; Oomori, Y.; Shimizu, Y.; Yamada, Y.; Kunisada, T. Neonatal lethality of neural crest cell-specific Rest knockout mice is associated with gastrointestinal distension caused by aberrations of myenteric plexus. Genes Cells 2014, 19, 723–742.
- Tomasoni, R.; Negrini, S.; Fiordaliso, S.; Klajn, A.; Tkatch, T.; Mondino, A.; Meldolesi, J.; D’Alessandro, R. A signaling loop of REST, TSC2 and beta-catenin governs proliferation and function of PC12 neural cells. J. Cell Sci. 2011, 124, 3174–3186.
- Toshiyuki, N.; Ichiro, M. Molecular mechanisms regulating cell type specific expression of BMP/RA Inducible Neural-specific Protein-1 that suppresses cell cycle progression: Roles of NRSF/REST and DNA methylation. Brain Res. Mol. Brain Res. 2004, 125, 47–59.
- Yoo, A.S.; Staahl, B.T.; Chen, L.; Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 2009, 460, 642–646.
- Formisano, L.; Guida, N.; Laudati, G.; Boscia, F.; Esposito, A.; Secondo, A.; Di Renzo, G.; Canzoniero, L.M. Extracellular signal-related kinase 2/specificity protein 1/specificity protein 3/repressor element-1 silencing transcription factor pathway is involved in Aroclor 1254-induced toxicity in SH-SY5Y neuronal cells. J. Neurosci. Res. 2015, 93, 167–177.
- Rivera-Cervantes, M.C.; Castaneda-Arellano, R.; Castro-Torres, R.D.; Gudino-Cabrera, G.; Feria y Velasco, A.I.; Camins, A.; Beas-Zarate, C. P38 MAPK inhibition protects against glutamate neurotoxicity and modifies NMDA and AMPA receptor subunit expression. J. Mol. Neurosci. 2015, 55, 596–608.
- Pajarillo, E.; Rizor, A.; Son, D.S.; Aschner, M.; Lee, E. The transcription factor REST up-regulates tyrosine hydroxylase and antiapoptotic genes and protects dopaminergic neurons against manganese toxicity. J. Biol. Chem. 2020, 295, 3040–3054.
- Kallunki, P.; Edelman, G.M.; Jones, F.S. Tissue-specific expression of the L1 cell adhesion molecule is modulated by the neural restrictive silencer element. J. Cell Biol. 1997, 138, 1343–1354.
- Schulte, C.; Racchetti, G.; D’Alessandro, R.; Meldolesi, J. A new form of neurite outgrowth sustained by the exocytosis of enlargeosomes expressed under the control of REST. Traffic 2010, 11, 1304–1314.
- Paquette, A.J.; Perez, S.E.; Anderson, D.J. Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 12318–12323.
- Schoch, S.; Cibelli, G.; Thiel, G. Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J. Biol. Chem. 1996, 271, 3317–3323.
- Chin, L.S.; Weigel, C.; Li, L. Transcriptional regulation of gene expression of sec6, a component of mammalian exocyst complex at the synapse. Brain Res. Mol. Brain Res. 2000, 79, 127–137.
- Pozzi, D.; Lignani, G.; Ferrea, E.; Contestabile, A.; Paonessa, F.; D’Alessandro, R.; Lippiello, P.; Boido, D.; Fassio, A.; Meldolesi, J.; et al. REST/NRSF-mediated intrinsic homeostasis protects neuronal networks from hyperexcitability. EMBO J. 2013, 32, 2994–3007.
- Kim, C.S.; Hwang, C.K.; Song, K.Y.; Choi, H.S.; Kim, D.K.; Law, P.Y.; Wei, L.N.; Loh, H.H. Novel function of neuron-restrictive silencer factor (NRSF) for posttranscriptional regulation. Biochim. Biophys. Acta 2008, 1783, 1835–1846.
- Kim, C.S.; Hwang, C.K.; Choi, H.S.; Song, K.Y.; Law, P.Y.; Wei, L.N.; Loh, H.H. Neuron-restrictive silencer factor (NRSF) functions as a repressor in neuronal cells to regulate the mu opioid receptor gene. J. Biol. Chem. 2004, 279, 46464–46473.
- Kim, C.S.; Choi, H.S.; Hwang, C.K.; Song, K.Y.; Lee, B.K.; Law, P.Y.; Wei, L.N.; Loh, H.H. Evidence of the neuron-restrictive silencer factor (NRSF) interaction with Sp3 and its synergic repression to the mu opioid receptor (MOR) gene. Nucleic Acids Res. 2006, 34, 6392–6403.
- Kong, M.; Shi, L.; Zhou, Y.; He, J.; Zhang, W.; Gu, X.; Zhang, J.; Ma, Z. Changes of Mu-opioid receptor and neuron-restrictive silencer factor in periaquductal gray in mouse models of remifentanil-induced postoperative hyperalgesia. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014, 39, 901–906.
- Shudo, Y.; Shimojo, M.; Fukunaga, M.; Ito, S. Pituitary adenylate cyclase-activating polypeptide is regulated by alternative splicing of transcriptional repressor REST/NRSF in nerve injury. Life Sci. 2015, 143, 174–181.
- Wang, D.; Yu, J. Negative regulation of REST on NR2B in spinal cord contributes to the development of bone cancer pain in mice. Oncotarget 2016, 7, 85564–85572.
- Zhu, C.; Tang, J.; Ding, T.; Chen, L.; Wang, W.; Mei, X.P.; He, X.T.; Wang, W.; Zhang, L.D.; Dong, Y.L.; et al. Neuron-restrictive silencer factor-mediated downregulation of mu-opioid receptor contributes to the reduced morphine analgesia in bone cancer pain. Pain 2017, 158, 879–890.
- Xiao-Die, X.; Xiao-Hong, W.; Cheng-Feng, H.; Zhong-Yu, Y.; Jian-Tao, W.; Hou-Guang, Z.; Jing-Chun, G. Increased NRSF/REST in anterior cingulate cortex contributes to diabetes-related neuropathic pain. Biochem. Biophys. Res. Commun. 2020, 527, 785–790.
- Zhang, J.; Chen, S.R.; Chen, H.; Pan, H.L. RE1-silencing transcription factor controls the acute-to-chronic neuropathic pain transition and Chrm2 receptor gene expression in primary sensory neurons. J. Biol. Chem. 2018, 293, 19078–19091.
- Zhang, F.; Gigout, S.; Liu, Y.; Wang, Y.; Hao, H.; Buckley, N.J.; Zhang, H.; Wood, I.C.; Gamper, N. Repressor element 1-silencing transcription factor drives the development of chronic pain states. Pain 2019, 160, 2398–2408.
- Li, Y.; Liu, Q.; Yang, Y.; Lv, Y.; Chen, L.; Bai, C.; Nan, X.; Wang, Y.; Pei, X. Regulatory role of neuron-restrictive silencing factor in the specific expression of cocaine- and amphetamine-regulated transcript gene. J. Neurochem. 2008, 106, 1314–1324.
- D’Alessandro, R.; Klajn, A.; Stucchi, L.; Podini, P.; Malosio, M.L.; Meldolesi, J. Expression of the neurosecretory process in PC12 cells is governed by REST. J. Neurochem. 2008, 105, 1369–1383.
- Prada, I.; Marchaland, J.; Podini, P.; Magrassi, L.; D’Alessandro, R.; Bezzi, P.; Meldolesi, J. REST/NRSF governs the expression of dense-core vesicle gliosecretion in astrocytes. J. Cell Biol. 2011, 193, 537–549.
- Valiya Veettil, M.; Krishna, G.; Roy, A.; Ghosh, A.; Dutta, D.; Kumar, B.; Chakraborty, S.; Anju, T.R.; Sharma-Walia, N.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells. J. Virol. 2020, 94, e01692-19.
- Rockowitz, S.; Zheng, D. Significant expansion of the REST/NRSF cistrome in human versus mouse embryonic stem cells: Potential implications for neural development. Nucleic Acids Res. 2015, 43, 5730–5743.
- Wang, P.; Zhao, D.; Rockowitz, S.; Zheng, D. Divergence and rewiring of regulatory networks for neural development between human and other species. Neurogenesis 2016, 3, e1231495.
- Bolton, J.L.; Schulmann, A.; Garcia-Curran, M.M.; Regev, L.; Chen, Y.; Kamei, N.; Shao, M.; Singh-Taylor, A.; Jiang, S.; Noam, Y.; et al. Unexpected Transcriptional Programs Contribute to Hippocampal Memory Deficits and Neuronal Stunting after Early-Life Adversity. Cell Rep. 2020, 33, 108511.
- Yang, Y.; Zhang, X.; Li, D.; Fang, R.; Wang, Z.; Yun, D.; Wang, M.; Wang, J.; Dong, H.; Fei, Z.; et al. NRSF regulates age-dependently cognitive ability and its conditional knockout in APP/PS1 mice moderately alters ad-like pathology. Hum. Mol. Genet. 2022, 32, 2558–2575.
- Yang, M.; Li, Y.; Hu, L.; Luo, D.; Zhang, Y.; Xiao, X.; Li, G.; Zhang, L.; Zhu, G. Lead exposure inhibits expression of SV2C through NRSF. Toxicology 2018, 398–399, 23–30.
- Verma, P.; Gupta, R.K.; Gandhi, B.S.; Singh, P. CDRI-08 Attenuates REST/NRSF-Mediated Expression of NMDAR1 Gene in PBDE-209-Exposed Mice Brain. Evid. Based Complement. Alternat Med. 2015, 2015, 403840.
- Mori, N.; Mizuno, T.; Murai, K.; Nakano, I.; Yamashita, H. Effect of age on the gene expression of neural-restrictive silencing factor NRSF/REST. Neurobiol. Aging 2002, 23, 255–262.
- Rocchi, A.; Carminati, E.; De Fusco, A.; Kowalska, J.A.; Floss, T.; Benfenati, F. REST/NRSF deficiency impairs autophagy and leads to cellular senescence in neurons. Aging Cell 2021, 20, e13471.
- Yang, P.; Sun, R.; Yao, M.; Chen, W.; Wang, Z.; Fei, J. A C-terminal truncated mutation of spr-3 gene extends lifespan in Caenorhabditis elegans. Acta Biochim. Biophys. Sin. 2013, 45, 540–548.
- Xue, W.J.; He, C.F.; Zhou, R.Y.; Xu, X.D.; Xiang, L.X.; Wang, J.T.; Wang, X.R.; Zhou, H.G.; Guo, J.C. High glucose and palmitic acid induces neuronal senescence by NRSF/REST elevation and the subsequent mTOR-related autophagy suppression. Mol. Brain 2022, 15, 61.
- Obata, H.; Naito, K.; Kikui, A.; Nakamura, S.; Suzuki, K.; Kawakita, S.; Suzuki, T.; Goto, K.; Nagura, N.; Sugiyama, Y.; et al. Age-related differences for expression of the nerve-specific proteins after peripheral nerve injury. Exp. Ther. Med. 2022, 24, 682.
- Kawamura, M.; Sato, S.; Matsumoto, G.; Fukuda, T.; Shiba-Fukushima, K.; Noda, S.; Takanashi, M.; Mori, N.; Hattori, N. Loss of nuclear REST/NRSF in aged-dopaminergic neurons in Parkinson’s disease patients. Neurosci. Lett. 2019, 699, 59–63.
- Yu, M.; Cai, L.; Liang, M.; Huang, Y.; Gao, H.; Lu, S.; Fei, J.; Huang, F. Alteration of NRSF expression exacerbating 1-methyl-4-phenyl-pyridinium ion-induced cell death of SH-SY5Y cells. Neurosci. Res. 2009, 65, 236–244.
- Yu, M.; Suo, H.; Liu, M.; Cai, L.; Liu, J.; Huang, Y.; Xu, J.; Wang, Y.; Zhu, C.; Fei, J.; et al. NRSF/REST neuronal deficient mice are more vulnerable to the neurotoxin MPTP. Neurobiol. Aging 2013, 34, 916–927.
- Suo, H.; Wang, P.; Tong, J.; Cai, L.; Liu, J.; Huang, D.; Huang, L.; Wang, Z.; Huang, Y.; Xu, J.; et al. NRSF is an essential mediator for the neuroprotection of trichostatin A in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2015, 99, 67–78.
- Huang, D.; Li, Q.; Wang, Y.; Liu, Z.; Wang, Z.; Li, H.; Wang, J.; Su, J.; Ma, Y.; Yu, M.; et al. Brain-specific NRSF deficiency aggravates dopaminergic neurodegeneration and impairs neurogenesis in the MPTP mouse model of Parkinson’s disease. Aging 2019, 11, 3280–3297.
- Li, H.; Liu, Z.; Wu, Y.; Chen, Y.; Wang, J.; Wang, Z.; Huang, D.; Wang, M.; Yu, M.; Fei, J.; et al. The deficiency of NRSF/REST enhances the pro-inflammatory function of astrocytes in a model of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165590.
- Zuccato, C.; Tartari, M.; Crotti, A.; Goffredo, D.; Valenza, M.; Conti, L.; Cataudella, T.; Leavitt, B.R.; Hayden, M.R.; Timmusk, T.; et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 2003, 35, 76–83.
- Marullo, M.; Valenza, M.; Mariotti, C.; Di Donato, S.; Cattaneo, E.; Zuccato, C. Analysis of the repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy of non-neuronal genes in peripheral lymphocytes from patients with Huntington’s disease. Brain Pathol. 2010, 20, 96–105.
- Schiffer, D.; Caldera, V.; Mellai, M.; Conforti, P.; Cattaneo, E.; Zuccato, C. Repressor element-1 silencing transcription factor (REST) is present in human control and Huntington’s disease neurones. Neuropathol. Appl. Neurobiol. 2014, 40, 899–910.
- Zuccato, C.; Belyaev, N.; Conforti, P.; Ooi, L.; Tartari, M.; Papadimou, E.; MacDonald, M.; Fossale, E.; Zeitlin, S.; Buckley, N.; et al. Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington’s disease. J. Neurosci. 2007, 27, 6972–6983.
- Shimojo, M. Huntingtin regulates RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) nuclear trafficking indirectly through a complex with REST/NRSF-interacting LIM domain protein (RILP) and dynactin p150 Glued. J. Biol. Chem. 2008, 283, 34880–34886.
- Datta, M.; Bhattacharyya, N.P. Regulation of RE1 protein silencing transcription factor (REST) expression by HIP1 protein interactor (HIPPI). J. Biol. Chem. 2011, 286, 33759–33769.
- Chen, G.L.; Ma, Q.; Goswami, D.; Shang, J.; Miller, G.M. Modulation of nuclear REST by alternative splicing: A potential therapeutic target for Huntington’s disease. J. Cell. Mol. Med. 2017, 21, 2974–2984.
- Navarrete-Modesto, V.; Orozco-Suarez, S.; Alonso-Vanegas, M.; Feria-Romero, I.A.; Rocha, L. REST/NRSF transcription factor is overexpressed in hippocampus of patients with drug-resistant mesial temporal lobe epilepsy. Epilepsy Behav. 2019, 94, 118–123.
- Hu, X.L.; Cheng, X.; Cai, L.; Tan, G.H.; Xu, L.; Feng, X.Y.; Lu, T.J.; Xiong, H.; Fei, J.; Xiong, Z.Q. Conditional deletion of NRSF in forebrain neurons accelerates epileptogenesis in the kindling model. Cereb. Cortex 2011, 21, 2158–2165.
- Liu, M.; Sheng, Z.; Cai, L.; Zhao, K.; Tian, Y.; Fei, J. Neuronal conditional knockout of NRSF decreases vulnerability to seizures induced by pentylenetetrazol in mice. Acta Biochim. Biophys. Sin. 2012, 44, 476–482.
- Patterson, K.P.; Barry, J.M.; Curran, M.M.; Singh-Taylor, A.; Brennan, G.; Rismanchi, N.; Page, M.; Noam, Y.; Holmes, G.L.; Baram, T.Z. Enduring Memory Impairments Provoked by Developmental Febrile Seizures Are Mediated by Functional and Structural Effects of Neuronal Restrictive Silencing Factor. J. Neurosci. 2017, 37, 3799–3812.
- McClelland, S.; Flynn, C.; Dubé, C.; Richichi, C.; Zha, Q.; Ghestem, A.; Esclapez, M.; Bernard, C.; Baram, T.Z. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann. Neurol. 2011, 70, 454–464.
- Carminati, E.; Buffolo, F.; Rocchi, A.; Michetti, C.; Cesca, F.; Benfenati, F. Mild Inactivation of RE-1 Silencing Transcription Factor (REST) Reduces Susceptibility to Kainic Acid-Induced Seizures. Front. Cell. Neurosci. 2019, 13, 580.
- Garriga-Canut, M.; Schoenike, B.; Qazi, R.; Bergendahl, K.; Daley, T.J.; Pfender, R.M.; Morrison, J.F.; Ockuly, J.; Stafstrom, C.; Sutula, T.; et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat. Neurosci. 2006, 9, 1382–1387.
- Hu, X.L.; Cheng, X.; Fei, J.; Xiong, Z.Q. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia 2011, 52, 1609–1616.
- Bassuk, A.G.; Wallace, R.H.; Buhr, A.; Buller, A.R.; Afawi, Z.; Shimojo, M.; Miyata, S.; Chen, S.; Gonzalez-Alegre, P.; Griesbach, H.L.; et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am. J. Hum. Genet. 2008, 83, 572–581.
- Quinn, J.P.; Bubb, V.J.; Marshall-Jones, Z.V.; Coulson, J.M. Neuron restrictive silencer factor as a modulator of neuropeptide gene expression. Regul. Pept. 2002, 108, 135–141.
- Spencer, E.M.; Chandler, K.E.; Haddley, K.; Howard, M.R.; Hughes, D.; Belyaev, N.D.; Coulson, J.M.; Stewart, J.P.; Buckley, N.J.; Kipar, A.; et al. Regulation and role of REST and REST4 variants in modulation of gene expression in in vivo and in vitro in epilepsy models. Neurobiol. Dis. 2006, 24, 41–52.
- Gillies, S.; Haddley, K.; Vasiliou, S.; Bubb, V.J.; Quinn, J.P. The human neurokinin B gene, TAC3, and its promoter are regulated by Neuron Restrictive Silencing Factor (NRSF) transcription factor family. Neuropeptides 2009, 43, 333–340.
- Hall, A.M.; Brennan, G.P.; Nguyen, T.M.; Singh-Taylor, A.; Mun, H.S.; Sargious, M.J.; Baram, T.Z. The Role of Sirt1 in Epileptogenesis. Eneuro 2017, preprint.
- Brennan, G.P.; Dey, D.; Chen, Y.; Patterson, K.P.; Magnetta, E.J.; Hall, A.M.; Dube, C.M.; Mei, Y.T.; Baram, T.Z. Dual and Opposing Roles of MicroRNA-124 in Epilepsy Are Mediated through Inflammatory and NRSF-Dependent Gene Networks. Cell Rep. 2016, 14, 2402–2412.
- Calderone, A.; Jover, T.; Noh, K.M.; Tanaka, H.; Yokota, H.; Lin, Y.; Grooms, S.Y.; Regis, R.; Bennett, M.V.; Zukin, R.S. Ischemic insults derepress the gene silencer REST in neurons destined to die. J. Neurosci. 2003, 23, 2112–2121.
- Formisano, L.; Noh, K.M.; Miyawaki, T.; Mashiko, T.; Bennett, M.V.; Zukin, R.S. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 4170–4175.
- Liang, H.M.; Geng, L.J.; Shi, X.Y.; Zhang, C.G.; Wang, S.Y.; Zhang, G.M. By up-regulating mu- and delta-opioid receptors, neuron-restrictive silencer factor knockdown promotes neurological recovery after ischemia. Oncotarget 2017, 8, 101012–101025.
- He, C.F.; Xue, W.J.; Xu, X.D.; Wang, J.T.; Wang, X.R.; Feng, Y.; Zhou, H.G.; Guo, J.C. Knockdown of NRSF Alleviates Ischemic Brain Injury and Microvasculature Defects in Diabetic MCAO Mice. Front. Neurol. 2022, 13, 869220.
- Zhang, J.; Wang, S.; Yuan, L.; Yang, Y.; Zhang, B.; Liu, Q.; Chen, L.; Yue, W.; Li, Y.; Pei, X. Neuron-restrictive silencer factor (NRSF) represses cocaine- and amphetamine-regulated transcript (CART) transcription and antagonizes cAMP-response element-binding protein signaling through a dual NRSE mechanism. J. Biol. Chem. 2012, 287, 42574–42587.
- Kaneko, N.; Hwang, J.Y.; Gertner, M.; Pontarelli, F.; Zukin, R.S. Casein kinase 1 suppresses activation of REST in insulted hippocampal neurons and halts ischemia-induced neuronal death. J. Neurosci. 2014, 34, 6030–6039.
- Loe-Mie, Y.; Lepagnol-Bestel, A.M.; Maussion, G.; Doron-Faigenboim, A.; Imbeaud, S.; Delacroix, H.; Aggerbeck, L.; Pupko, T.; Gorwood, P.; Simonneau, M.; et al. SMARCA2 and other genome-wide supported schizophrenia-associated genes: Regulation by REST/NRSF, network organization and primate-specific evolution. Hum. Mol. Genet. 2010, 19, 2841–2857.
- Gulchina, Y.; Xu, S.J.; Snyder, M.A.; Elefant, F.; Gao, W.J. Epigenetic mechanisms underlying NMDA receptor hypofunction in the prefrontal cortex of juvenile animals in the MAM model for schizophrenia. J. Neurochem. 2017, 143, 320–333.
- Patel, P.D.; Bochar, D.A.; Turner, D.L.; Meng, F.; Mueller, H.M.; Pontrello, C.G. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron restrictive silencing factor (REST/NRSF) binding motif. J. Biol. Chem. 2007, 282, 26717–26724.
- Ishii, T.; Hashimoto, E.; Ukai, W.; Tateno, M.; Yoshinaga, T.; Saito, S.; Sohma, H.; Saito, T. Lithium-induced suppression of transcription repressor NRSF/REST: Effects on the dysfunction of neuronal differentiation by ethanol. Eur. J. Pharmacol. 2008, 593, 36–43.
- Warburton, A.; Savage, A.L.; Myers, P.; Peeney, D.; Bubb, V.J.; Quinn, J.P. Molecular signatures of mood stabilisers highlight the role of the transcription factor REST/NRSF. J. Affect. Disord. 2015, 172, 63–73.
- Henriksson, R.; Backman, C.M.; Harvey, B.K.; Kadyrova, H.; Bazov, I.; Shippenberg, T.S.; Bakalkin, G. PDYN, a gene implicated in brain/mental disorders, is targeted by REST in the adult human brain. Biochim. Biophys. Acta 2014, 1839, 1226–1232.
- Korosi, A.; Shanabrough, M.; McClelland, S.; Liu, Z.W.; Borok, E.; Gao, X.B.; Horvath, T.L.; Baram, T.Z. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 2010, 30, 703–713.
- Hai-Ying, C.; Nagano, K.; Ezzikouri, S.; Yamaguchi, C.; Kayesh, M.E.; Rebbani, K.; Kitab, B.; Nakano, H.; Kouji, H.; Kohara, M.; et al. Establishment of an intermittent cold stress model using Tupaia belangeri and evaluation of compound C737 targeting neuron-restrictive silencer factor. Exp. Anim. 2016, 65, 285–292.
- Singh-Taylor, A.; Molet, J.; Jiang, S.; Korosi, A.; Bolton, J.L.; Noam, Y.; Simeone, K.; Cope, J.; Chen, Y.; Mortazavi, A.; et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatry 2018, 23, 648–657.
- Wen, X.H.; Guo, Q.L.; Guo, J.C. Effect of 9—PAHSA on cognitive dysfunction in diabetic mice and its possible mechanism. Biochem. Biophys. Res. Commun. 2020, 524, 525–532.
- Kuwahara, K.; Saito, Y.; Takano, M.; Arai, Y.; Yasuno, S.; Nakagawa, Y.; Takahashi, N.; Adachi, Y.; Takemura, G.; Horie, M.; et al. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. EMBO J. 2003, 22, 6310–6321.
- Kuratomi, S.; Kuratomi, A.; Kuwahara, K.; Ishii, T.M.; Nakao, K.; Saito, Y.; Takano, M. NRSF regulates the developmental and hypertrophic changes of HCN4 transcription in rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007, 353, 67–73.
- Schweizer, P.A.; Yampolsky, P.; Malik, R.; Thomas, D.; Zehelein, J.; Katus, H.A.; Koenen, M. Transcription profiling of HCN-channel isotypes throughout mouse cardiac development. Basic. Res. Cardiol. 2009, 104, 621–629.
- Zhang, D.; Wu, B.; Wang, P.; Wang, Y.; Lu, P.; Nechiporuk, T.; Floss, T.; Greally, J.M.; Zheng, D.; Zhou, B. Non-CpG methylation by DNMT3B facilitates REST binding and gene silencing in developing mouse hearts. Nucleic Acids Res. 2017, 45, 3102–3115.
- Liu, J.; Liu, S.; Gao, H.; Han, L.; Chu, X.; Sheng, Y.; Shou, W.; Wang, Y.; Liu, Y.; Wan, J.; et al. Genome-wide studies reveal the essential and opposite roles of ARID1A in controlling human cardiogenesis and neurogenesis from pluripotent stem cells. Genome Biol. 2020, 21, 169.
- D’Souza, A.; Bucchi, A.; Johnsen, A.B.; Logantha, S.J.; Monfredi, O.; Yanni, J.; Prehar, S.; Hart, G.; Cartwright, E.; Wisloff, U.; et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Commun. 2014, 5, 3775.
- Koyama, R.; Mannic, T.; Ito, J.; Amar, L.; Zennaro, M.C.; Rossier, M.F.; Maturana, A.D. MicroRNA-204 Is Necessary for Aldosterone-Stimulated T-Type Calcium Channel Expression in Cardiomyocytes. Int. J. Mol. Sci. 2018, 19, 2941.
- Rossier, M.F. The Cardiac Mineralocorticoid Receptor (MR): A Therapeutic Target Against Ventricular Arrhythmias. Front. Endocrinol. 2021, 12, 694758.
- Somekawa, S.; Imagawa, K.; Naya, N.; Takemoto, Y.; Onoue, K.; Okayama, S.; Takeda, Y.; Kawata, H.; Horii, M.; Nakajima, T.; et al. Regulation of aldosterone and cortisol production by the transcriptional repressor neuron restrictive silencer factor. Endocrinology 2009, 150, 3110–3117.
- Inazumi, H.; Kuwahara, K.; Nakagawa, Y.; Kuwabara, Y.; Numaga-Tomita, T.; Kashihara, T.; Nakada, T.; Kurebayashi, N.; Oya, M.; Nonaka, M.; et al. NRSF-GNAO1 Pathway Contributes to the Regulation of Cardiac Ca2+ Homeostasis. Circ. Res. 2022, 130, 234–248.
- Atouf, F.; Czernichow, P.; Scharfmann, R. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J. Biol. Chem. 1997, 272, 1929–1934.
- Martin, D.; Kim, Y.H.; Sever, D.; Mao, C.A.; Haefliger, J.A.; Grapin-Botton, A. REST represses a subset of the pancreatic endocrine differentiation program. Dev. Biol. 2015, 405, 316–327.
- Kemp, D.M.; Lin, J.C.; Habener, J.F. Regulation of Pax4 paired homeodomain gene by neuron-restrictive silencer factor. J. Biol. Chem. 2003, 278, 35057–35062.
- Martin, D.; Tawadros, T.; Meylan, L.; Abderrahmani, A.; Condorelli, D.F.; Waeber, G.; Haefliger, J.A. Critical role of the transcriptional repressor neuron-restrictive silencer factor in the specific control of connexin36 in insulin-producing cell lines. J. Biol. Chem. 2003, 278, 53082–53089.
- Campa, D.; Capurso, G.; Pastore, M.; Talar-Wojnarowska, R.; Milanetto, A.C.; Landoni, L.; Maiello, E.; Lawlor, R.T.; Malecka-Panas, E.; Funel, N.; et al. Common germline variants within the CDKN2A/2B region affect risk of pancreatic neuroendocrine tumors. Sci. Rep. 2016, 6, 39565.
- Li, B.; Wang, S.; Liu, H.; Liu, D.; Zhang, J.; Zhang, B.; Yao, H.; Lv, Y.; Wang, R.; Chen, L.; et al. Neuronal restrictive silencing factor silencing induces human amniotic fluid-derived stem cells differentiation into insulin-producing cells. Stem Cells Dev. 2011, 20, 1223–1231.
- Li, H.T.; Jiang, F.X.; Shi, P.; Zhang, T.; Liu, X.Y.; Lin, X.W.; Pang, X.N. In vitro reprogramming of rat bone marrow-derived mesenchymal stem cells into insulin-producing cells by genetically manipulating negative and positive regulators. Biochem. Biophys. Res. Commun. 2012, 420, 793–798.
- Kurschat, P.; Bielenberg, D.; Rossignol-Tallandier, M.; Stahl, A.; Klagsbrun, M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J. Biol. Chem. 2006, 281, 2721–2729.
- Aoki, H.; Hara, A.; Kunisada, T. White spotting phenotype induced by targeted REST disruption during neural crest specification to a melanocyte cell lineage. Genes Cells 2015, 20, 439–449.
- Lee, H.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Regulation of mGluR1 expression in human melanocytes and melanoma cells. Biochim. Biophys. Acta 2012, 1819, 1123–1131.
- Aoki, H.; Ogino, H.; Tomita, H.; Hara, A.; Kunisada, T. Disruption of Rest Leads to the Early Onset of Cataracts with the Aberrant Terminal Differentiation of Lens Fiber Cells. PLoS ONE 2016, 11, e0163042.
- Tsuda, L.; Kaido, M.; Lim, Y.M.; Kato, K.; Aigaki, T.; Hayashi, S. An NRSF/REST-like repressor downstream of Ebi/SMRTER/Su(H) regulates eye development in Drosophila. EMBO J. 2006, 25, 3191–3202.
- Cheong, A.; Bingham, A.J.; Li, J.; Kumar, B.; Sukumar, P.; Munsch, C.; Buckley, N.J.; Neylon, C.B.; Porter, K.E.; Beech, D.J.; et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol. Cell 2005, 20, 45–52.
- Tharp, D.L.; Bowles, D.K. KCa3.1 Inhibition Decreases Size and Alters Composition of Atherosclerotic Lesions Induced by Low, Oscillatory Flow. Artery Res. 2021, 27, 93–100.
- Nishimura, E.; Sasaki, K.; Maruyama, K.; Tsukada, T.; Yamaguchi, K. Decrease in neuron-restrictive silencer factor (NRSF) mRNA levels during differentiation of cultured neuroblastoma cells. Neurosci. Lett. 1996, 211, 101–104.
- Lepagnol-Bestel, A.M.; Maussion, G.; Ramoz, N.; Moalic, J.M.; Gorwood, P.; Simonneau, M. Nrsf silencing induces molecular and subcellular changes linked to neuronal plasticity. NeuroReport 2007, 18, 441–446.
- Pandey, G.K.; Mitra, S.; Subhash, S.; Hertwig, F.; Kanduri, M.; Mishra, K.; Fransson, S.; Ganeshram, A.; Mondal, T.; Bandaru, S.; et al. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell 2014, 26, 722–737.
- Conti, L.; Crisafulli, L.; Caldera, V.; Tortoreto, M.; Brilli, E.; Conforti, P.; Zunino, F.; Magrassi, L.; Schiffer, D.; Cattaneo, E. REST controls self-renewal and tumorigenic competence of human glioblastoma cells. PLoS ONE 2012, 7, e38486.
- Ren, H.; Gao, Z.; Wu, N.; Zeng, L.; Tang, X.; Chen, X.; Liu, Z.; Zhang, W.; Wang, L.; Li, Z. Expression of REST4 in human gliomas in vivo and influence of pioglitazone on REST in vitro. Biochem. Biophys. Res. Commun. 2015, 463, 504–509.
- Dobson, T.H.W.; Tao, R.H.; Swaminathan, J.; Maegawa, S.; Shaik, S.; Bravo-Alegria, J.; Sharma, A.; Kennis, B.; Yang, Y.; Callegari, K.; et al. Transcriptional repressor REST drives lineage stage-specific chromatin compaction at Ptch1 and increases AKT activation in a mouse model of medulloblastoma. Sci. Signal 2019, 12, eaan8680.
- Su, X.; Gopalakrishnan, V.; Stearns, D.; Aldape, K.; Lang, F.F.; Fuller, G.; Snyder, E.; Eberhart, C.G.; Majumder, S. Abnormal expression of REST/NRSF and Myc in neural stem/progenitor cells causes cerebellar tumors by blocking neuronal differentiation. Mol. Cell. Biol. 2006, 26, 1666–1678.
- Lawinger, P.; Venugopal, R.; Guo, Z.S.; Immaneni, A.; Sengupta, D.; Lu, W.; Rastelli, L.; Marin Dias Carneiro, A.; Levin, V.; Fuller, G.N.; et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat. Med. 2000, 6, 826–831.
- Fuller, G.N.; Su, X.; Price, R.E.; Cohen, Z.R.; Lang, F.F.; Sawaya, R.; Majumder, S. Many human medulloblastoma tumors overexpress repressor element-1 silencing transcription (REST)/neuron-restrictive silencer factor, which can be functionally countered by REST-VP16. Mol. Cancer Ther. 2005, 4, 343–349.
- Coulson, J.M.; Edgson, J.L.; Woll, P.J.; Quinn, J.P. A splice variant of the neuron-restrictive silencer factor repressor is expressed in small cell lung cancer: A potential role in derepression of neuroendocrine genes and a useful clinical marker. Cancer Res. 2000, 60, 1840–1844.
- Gurrola-Diaz, C.; Lacroix, J.; Dihlmann, S.; Becker, C.M.; von Knebel Doeberitz, M. Reduced expression of the neuron restrictive silencer factor permits transcription of glycine receptor alpha1 subunit in small-cell lung cancer cells. Oncogene 2003, 22, 5636–5645.
- Neumann, S.B.; Seitz, R.; Gorzella, A.; Heister, A.; Doeberitz, M.; Becker, C.M. Relaxation of glycine receptor and onconeural gene transcription control in NRSF deficient small cell lung cancer cell lines. Brain Res. Mol. Brain Res. 2004, 120, 173–181.
- Chang, L.; Schwarzenbach, H.; Meyer-Staeckling, S.; Brandt, B.; Mayr, G.W.; Weitzel, J.M.; Windhorst, S. Expression Regulation of the Metastasis-Promoting Protein InsP3-Kinase-A in Tumor Cells. Mol. Cancer Res. 2011, 9, 497–506.
- Kreisler, A.; Strissel, P.L.; Strick, R.; Neumann, S.B.; Schumacher, U.; Becker, C.M. Regulation of the NRSF/REST gene by methylation and CREB affects the cellular phenotype of small-cell lung cancer. Oncogene 2010, 29, 5828–5838.
- Yoshida, M.; Oda, C.; Mishima, K.; Tsuji, I.; Obika, S.; Shimojo, M. An antisense amido-bridged nucleic acid gapmer oligonucleotide targeting SRRM4 alters REST splicing and exhibits anti-tumor effects in small cell lung cancer and prostate cancer cells. Cancer Cell Int. 2023, 23, 8.
- Wang, K.; Xu, K.; Leng, X.; Han, Y.; Fang, Q. miRNA-9 Inhibits Proliferation and Migration of Lung Squamous Cell Carcinoma Cells by Regulating NRSF/EGFR. Technol. Cancer Res. Treat. 2020, 19, 1533033820945807.
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C.; et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017, 545, 360–364.
- Moss, A.C.; Jacobson, G.M.; Walker, L.E.; Blake, N.W.; Marshall, E.; Coulson, J.M. SCG3 transcript in peripheral blood is a prognostic biomarker for REST-deficient small cell lung cancer. Clin. Cancer Res. 2009, 15, 274–283.
