Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Horticulture
Grape fruits are used for various purposes, including fresh consumption, juice extraction, drying, and wine production, with rich nutritional value. Tartaric acid (TA) is the primary organic acid present in grapes and a fundamental constituent of wine, responsible for shaping its taste, aroma, and overall quality.
- grape
- tartaric acid
- ascorbic acid
- metabolism
1. Introduction
Grapes (Vitis vinifera L.) are one of the oldest fruit crops globally, with the broadest cultivation history, largest planting area, and highest economic value. Grape fruits are used for various purposes, including fresh consumption, juice extraction, drying, and wine production, with rich nutritional value. China ranks first in grape production globally (FAO, 2021), which is currently transitioning from a focus on total growth to a more quality-oriented approach to production [1,2]. Grapes are one of the four major fruit crops with high adaptability to diverse soil and climatic conditions, early fruiting, high yield efficiency, and ease of cultivation, making it a popular choice among cultivators. Malic acids (MA) and tartaric acids (TA) are recognized as the primary organic acids in grape fruits, with TA serving as a distinctive feature of grapes, accounting for 42.8–77% of the organic acid content [3]. Therefore, TA is considered the fundamental component of grape fruit.
TA is a vital contributor to the acidity of the wine, which is not very strong, refreshing, and firm. In addition, it is relatively stable and remains unmetabolized during the winemaking process [4]. TA contributes to the unique flavor and low pH of the wine, thereby determining its resistance to spoilage, microbial stability, and aging potential. Despite the accumulation of TA being less susceptible to environmental influences, little is known about its synthesis in grape tissues and its exact location.
Grape varieties with a high concentration of TA are deemed suitable for winemaking due to their ability to enhance color stability, prevent oxidation, and inhibit spoilage [5]. Conversely, grape germplasm with low TA results in dull and thin wine that is susceptible to cloudy wines. Hence, achieving an optimal increase in organic acid content is essential in the winemaking process to produce wines that are full-bodied and smooth [5,6]. However, with the rise in temperatures caused by global warming, the acidity of wine grapes is decreasing. As a result, there is an increase in the pH of both the juice and the fermented wine, which can lead to a deterioration of microbial homeostasis. Thus, it needs more SO2 additions to effectively inhibit the growth of harmful microorganisms, but it will lead to excessive SO2 content in wine, affecting the flavor and aroma of wine [7]. To enhance fermented wine acidity and restore microbial homeostasis, winemakers often need to add significant amounts of tartaric acid during the winemaking process. This addition serves the purpose of regulating pH and TA levels, ultimately ensuring the desired quality of the wine [8,9]. The artificial addition of TA to maintain the acidity of wine increases the production cost of wineries. Therefore, the endogenous TA content of grapes plays a vital role in the quality of the wine. Grapes accumulate a large amount of TA during fruit development, prolonging hanging fruit time, promoting sugar accumulation, and increasing flavor substances while also enhancing wine stability and taste.
2. Characteristics of TA
2.1. Structure and Properties
Tartaric acid (TA) is a dicarboxylic acid with the molecular formula C4H6O6, also known as 2,3-dihydroxysuccinic acid or grape acid. In its unpurified form extracted from grapes, it exhibits its natural color, while purified TA appears as a white crystalline powder [10]. The acidity of TA is approximately 1.2 to 1.3 times higher than that of citric acid (CA) at the same concentration [3]. Tartaric acid is soluble in both water and ethanol. In grapes, the accumulated TA is primarily L-(R,R)-(+)-tartaric acid, which is also known as dextrotartaric acid. The mirror image, enantiomeric form is D-(S,S)-(-)-tartaric acid, or laevotartaric acid. TA can also exist in an optically inactive form called meso-(R, S)-tartaric acid, or mesotartaric acid (Figure 1). Another optically inactive form is DL(S,S/R,R)-(-)-tartaric acid, which is a 1:1 mixture of the laevo and dextro forms, known as racemic acid or paratartrate [10]. Organic acids play a crucial role in the wine aging process [11], and among them, TA is the most dominant one. TA levels are known to be less sensitive to climatic conditions during grape ripening [12]. A higher concentration of TA in grapes is associated with better resistance to the effects of climate change. However, it is important to note that climate change can also have a significant impact on the concentration of various organic acids in grapes [13,14].
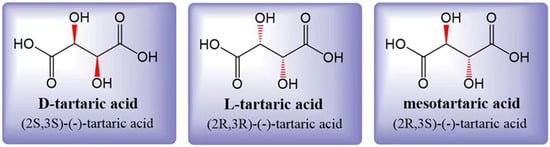
Figure 1. Three structural formulas for tartaric acid: laevotartaric acid (D-tartaric), dextrotartaric acid (L-tartaric), and mesotartaric acid. The dextro- and laevo- prefixes identify the (+) and (-) forms, respectively. Figure modified from [10].
2.2. Distribution and Transportation of TA
TA is primarily found in the form of potassium salt in a diverse range of plants and fruits, with only small amounts present in its free state. The accumulation of TA is known to occur during the early stages of leaf and berry development, with limited biosynthesis occurring in other tissues and mature berries [15]. In grape berries, TA levels (TA content measured in grams) gradually increase during fruit growth and development, peaking during veraison, and then decrease during the ripening process. This accumulation pattern is similar to that observed for other organic acids. The content of TA in grapes varies depending on the genotype, with wine grape varieties generally exhibiting higher TA content than fresh grapes [16].
Early research has suggested that tartrate mainly originates from leaves [17]. Currently, available studies indicate that besides grape leaves, leaves from approximately 15 other species listed in Buch’s bibliography of Organic Acids in Higher Plants also contain TA [18]. While tartrate can be found in microscopic amounts in widely distributed angiosperms, it accumulates significantly only in three different families: Vitaceae, Geraniaceae, and Leguminosae [18]. A conducted study exposed grapevine leaves to 14CO2 light and dark conditions for different durations, followed by radio labeling, and found that TA is present in the leaves at four times the concentration of MA [19]. Moreover, it has been suggested that leaves are the primary site of TA synthesis in grapes, and the accumulation of TA in grape berries is derived via translocation [20,21].
The organic matter is synthesized by leaves through photosynthesis, which is then decomposed, supplied, and stored in the form of sucrose in berry pre-veraison. A proportion of sucrose is converted into fructose and glucose to participate in glycolysis during respiration. Sucrose subsequently becomes the primary raw material for ATP synthesis, allowing for the synthesis of tartaric acid in berries. Organic acids are primarily transported across the tonoplast in plants. For example, malate and citrate accumulate in plant cells due to their complex metabolism and vacuolar storage and are transported into the vacuole occurring by facilitated diffusion [22,23]. Facilitated diffusion is the primary mode of transport of organic acids from the cytosol to the vacuole; this transportation process is mediated mainly by channels, carriers, and proton pumps [24]. Research has shown that AsA, a monovalent anion at physiological pHs, is unable to permeate membranes and must be transported through chloroplasts, apoplasts, and vacuoles [25]. Previous studies have detected AsA in the phloem of various crops such as barley, peas, potatoes, tobacco, and turnips [26]. Burbidge (2021) et al. believed that AsA is transported through the phloem to the leaves and fruits [8]. It is believed that the biosynthesis of TA occurs in the cytoplasm and this hypothesis is supported by the fact that the cytoplasm contains high concentrations of AsA [27]. Moreover, L-Idonate dehydrogenase (L-IdnDH) is the only known enzyme of the TA biosynthetic pathway in grape berries, which is distributed mainly in the vacuole (immature berries) and cytoplasm (mature berries) [28]. On the other hand, Ford et al. (2012) claimed the accumulation of TA occurs in the vacuoles of mature berries, which is synthesized starting from the earliest stages of berry formation and continuing until 40–50 days after flowering [29]. Hence, the distribution of TA is closely related to berry maturity. In immature berries, TA is mainly synthesized in the cytoplasm, while in mature berries, it is primarily stored in the vacuole. Again, it is believed that AsA is the precursor of TA synthesis, the accumulation of TA in the vacuole is related to the transport of AsA, and the biosynthesis of AsA in leaves and its eventual transport to grape berries provide a regulatory process for the location and timing of TA synthesis in grapes.
3. The Biosynthetic Pathway of TA
Table fruits are typically rich in AsA, but grapes do not accumulate large amounts of it as it is used as a precursor for the synthesis of TA [30]. While there is interest in the accumulation of TA in grapes, few mechanisms are known for the regulation of TA concentration. The three pathways of TA biosynthesis in higher plants are AsA C4/C5, AsA C2/C3, and 5-keto-D-gluconate C4/C5 sites cleavage leading to TA formation [8]. In grape berries, AsA is cleaved between C4 and C5 to form 2-keto-L-gulonic acid (2KGA), which is then converted to L-Idonic acid, followed by 5-keto-D-gluconic acid (5KGA), L-Threo-tetruronate acid, and, ultimately, converted into TA [29]. The synthetic pathway of TA in rose-scented geranium (Pelargonium sp.) involves the cleavage reaction of ascorbic acid C2 and C3 to generate oxalic acid (OA) and threonic acid, followed by conversion of threonic acid to TA [31]. There are two distinct stages of TA biosynthesis in grape berries: the synthesis of AsA, the precursor of TA, and the synthesis of TA (Figure 2).
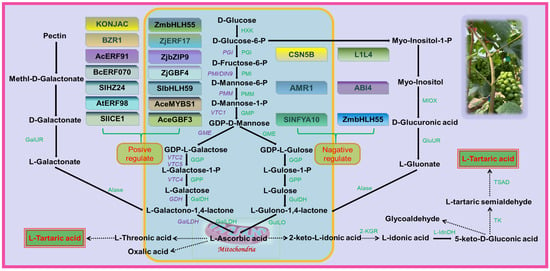
Figure 2. Grape involved in tartaric acid biosynthesis pathway. HXK, Hexokinase; PGI, Glucose-6-phosphate isomerase; PMI, Mannose-6-phosphate isomerase; PMM, Phospho mannomutase; GMP, GDP-Mannose pyrophosphorylase; GME, GDP-D-mannose-3′, 5′-epimerase; GGP, GDP-L-galactose phosphorylase; GalDH, L-galactose dehydrogenase; GulDH, L-gulose dehydrogenase; GulLO, L-Gulono-1,4-lactone oxidase; GalLDH, L-galactono-1,4-lactone dehydrogenase; GalUR, D-galacturonate reductase; GluUR, D-glucuronate reductase Alase, Aldonolactonase; MIOX, Myo-inositol oxygenase; 2-KGR, 2-keto-L-gulonate reductase; L-IdnDH, L-Idonate dehydrogenase; TK, Transketolase; TSAD, Tartaric semialdehyde dehydrogenase. The solid lines in the figure indicate that the pathways are confirmed and the dashed lines indicate that they are unconfirmed. The figure identifies several transcription factors that are involved in regulating the biosynthesis of AsA. Some transcription factors, such as SlICE1, AtERF98, SlHZ24, and BZR1, have been shown to have a positive effect on AsA biosynthesis. On the other hand, other transcription factors, including SlNFYA10, ABI4, CNSN5B, ZmbHLH55, AMR1, and L1L4, have a negative regulatory effect on AsA biosynthesis. Figure modified from [30,32,33].
3.1. AsA Biosynthesis Stage
There are several proposed pathways for AsA synthesis in plants, of which the L-galactose pathway is the predominant one in plants [32] and also an important pathway in grape berries [33], while alternative pathways for AsA have been proposed, including the L-gulose pathway [34], D-galacturonate pathway [35], and myo-inositol pathway [36]. In the L-galactose pathway, GDP-D-mannose is catalyzed by GDP-mannose-3′,5′-differential isomerase (GME) to produce GDP-L-galactose, which is then converted by GDP-L-galactose phosphorylase (VTC2) into L-galactose-1-P. L-galactose is then generated by L-galactose-1-P-phosphorylase (VTC4) before being dehydrogenated by L-galactate dehydrogenase (L-GalDH) to produce L-galactono-1,4-lactone, which is the immediate precursor of AsA. Finally, L-galactono-1,4-lactone is catalyzed by L-galactono-1,4-lactone dehydrogenase (L-GalLDH) to generate AsA [37,38].
3.2. TA Biosynthesis Stage
Based on previous research, it is postulated that the biosynthesis of TA in plants involves the hydrolysis and oxidation of AsA to 2-KGA, which is subsequently reduced to L-Idonic acid by the enzyme 2-keto-L-gulonic acid reductase (2-KGR). L-Idonic acid is then oxidized by L-Idonic acid dehydrogenase (L-IdnDH) to 5-KGA, which is cleaved between C4 and C5 to form 4-carbon L-threo-tetruronate. This compound is further oxidized to yield TA [39]. However, the specific enzymes involved in the biosynthesis of TA in plants, including the functions of transketolase (TK) and tartaric acid semialdehyde dehydrogenase (TSAD) in the grapevine TA synthesis pathway, remain unverified and require in-depth investigation.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9111173
This entry is offline, you can click here to edit this entry!
