Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Anthocyanins are a type of flavonoids that give plants and fruits their vibrant colors. They are known for their potent antioxidant properties and have been linked to various health benefits. Due to their ability to modulate mechanisms implicated in the onset of neurological diseases, anthocyanins hold significant potential for treating such conditions.
- anthocyanins
- antioxidants
- food chemicals
- natural products
- neuroprotection
- neurodegeneration
1. Anthocyanins
1.1. Dietary Sources
Anthocyanins constitute the largest group of water-soluble pigments, contributing shades of pink, red, blue, or purple to the vacuolar sap of flowers and fruits’ epidermal tissues [28,29]. These compounds can also exist in colorless forms depending on pH levels. In their aglycone state (anthocyanidins), they exhibit instability. Although they resist light-induced degradation in plants, they are vulnerable to pH fluctuations and oxidative conditions. Glycosylation, often with glucose at the 3-position, and esterification with organic or phenolic acids prevent degradation. Additionally, complex formation with other flavonoids stabilizes anthocyanins [30].
Anthocyanins are present in red wine, some cereal varieties, and select leafy vegetables and roots (e.g., eggplants, cabbage, beans, onions, radishes). Red wine contains approximately 200–350 mg of anthocyanins per liter, with these compounds undergoing structural transformations as the wine matures [1]. However, fruits remain the primary source of anthocyanin consumption [31]. While mainly concentrated in the peel, certain red fruits, like cherries and strawberries, also contain anthocyanins in the pulp. Cyanidin (Figure 1B) stands out as the most prevalent anthocyanidin in foods [32]. Levels typically correlate with color intensity, with values reaching up to 2 to 4 g/kg of fresh weight in blackcurrants or blackberries, increasing as the fruits ripen [33].
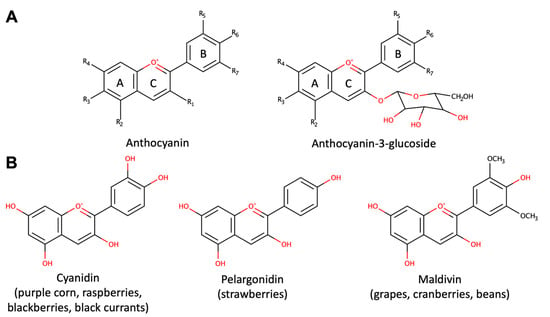
Figure 1. Structure of anthocyanins. (A) The general structures of anthocyanins and anthocyanins-3-glucoside. (B) Structures of bioactive anthocyanins with their common dietary sources.
1.2. Chemistry and Its Relationship with Bioavailability and Biodistribution
Anthocyanins exhibit a complex structure, characterized by glycosylation, polyhydroxy or polymethoxylated 2-phenylbenzopyryl derivatives, featuring two benzoyl rings (A and B) separated by a heterocyclic ring (C) (Figure 1A). The plant kingdom boasts around 400 distinct anthocyanins [29]. These flavonoids are distinguished by their heightened oxidation state, boasting a fully unsaturated C ring and a hydroxyl group at the 3-position [34]. Anthocyanins consist of an anthocyanidin (aglycone) bound to sugars (Figure 1A), often accompanied by organic acids in the case of acylated anthocyanins. Notably, variations in the number of sugars and binding positions, as well as the acylating groups of sugar substitutions, contribute to the diverse array of anthocyanin structures. Acylated anthocyanins exhibit pH-stable characteristics, tending to exhibit bluer hues compared to non-acylated counterparts. Favorable storage conditions involve cool, dark environments to maintain anthocyanin integrity due to the impact of light and temperature on anthocyanin degradation [34].
The therapeutic effects of anthocyanins require their absorption from dietary sources. Anthocyanins are susceptible to degradation in human saliva [35] and by the intestinal microbiota [36]. Due to their hydrophilicity, anthocyanins do not cross the gastrointestinal epithelium for paracellular absorption but use transporters. Anthocyanin glycosides can be translocated by sodium-dependent glucose transporter 1 (SGLT1), glucose transporter 2 (GLUT2), and organic anion-transporting polypeptide 2B1 (OATP 2B1), whereas the aglycon forms use only GLUT2 and OATP 2B1 [37]. After gastrointestinal absorption, anthocyanins are susceptible to phase II biotransformation, and their metabolites are transported in an ATP-dependent manner [38]. Because of all the above, some authors have expressed concerns that the therapeutic efficacy of anthocyanins may be hindered by their low bioavailability.
Hahm and collaborators [37] recently summarized multiple in vivo pharmacokinetic studies with anthocyanins, finding that, in general, bioavailability is low. However, the physicochemical properties of each anthocyanin influence the bioavailability. For example, the presence of cationic groups within anthocyanin glycosides appears to render them resistant to enzymatic conjugation, facilitating efficient and rapid absorption as glycosides both in experimental animals and humans [39,40,41,42]. Anthocyanins like cyanidin-3-glucoside and cyanidin-3,5-diglucoside from fruits are incorporated into the liver and plasma of rats and humans, indicating that structurally intact glycoside forms of anthocyanins are efficiently absorbed from the digestive tract into the bloodstream [43,44]. In addition, the type of sugar bound to anthocyanins has been identified as an influential factor in their permeability and bioavailability [45].
Importantly, a growing body of evidence underscores the ability of anthocyanins to traverse the BBB [46,47]. Anthocyanins and their derivatives, such as cyanidin-3-rutinoside and pelargonidin-3-O-glucoside, are taken up by mouse and rat brain endothelial cells, revealing their capacity to penetrate the BBB [48]. Intravenous injection of anthocyanin cyanidin-3-O-b-D-glucoside further supports rapid brain uptake [49]. Andres-Lacueva et al. (2005) found anthocyanins like cyanidin-3-O-b-D-galactoside, cyanidin-3-O-b-D-glucoside, and cyanidin-3-O-b-D-arabinose in various brain regions of rats fed blueberry polyphenols, suggesting that dietary supplementation allows direct brain access [50]. Yet, the possible therapeutic use of anthocyanins in neurodegenerative diseases requires further research to identify strategies for improving bioavailability and biodistribution of anthocyanins with specific activities.
2. Alzheimer’s Disease
The protective impact of anthocyanins against AD is underscored by their potential to delay disease progression. Consistent with this, studies reveal that consistent consumption of fruits, vegetables, and beverages like green tea and red wine (in moderation) diminishes the risk of age-related neurological disorders, including AD [124,125]. Examination of data on AD prevalence and incidence in relation to genetic and environmental factors suggests that the use of antioxidant supplements correlates with reduced occurrence of AD [126].
In animal models, anthocyanins have demonstrated AD-delaying effects. For instance, in a mutant AD mouse model, anthocyanins from blueberry and black currant impeded Aβ deposition and mitigated cognitive impairment [127]. Cranberry anthocyanins avert memory and learning deficits in rats induced by streptozotocin injection by regulating ion pumps and cholinergic neurotransmission [128]. Anthocyanins in gold nanoparticles alleviate memory loss and neurodegeneration in mice with Alzheimer’s-like symptoms by reducing Aβ, beta-secretase (BACE-1), and amyloid precursor protein levels [113]. Administration of cyanidin-3-glucoside orally halted cognitive decline induced by Aβ peptide [129].
Mechanistically, the effects of anthocyanins in AD mouse models relate to changes in Aβ deposition. Anthocyanin mixtures hinder Aβ oligomerization and subsequent tau phosphorylation, potentially curbing tau protein aggregate formation [130]. Cranberry-derived anthocyanin-rich extracts hinder Aβ1-40 and Aβ1-42 peptide formation in vitro, diverting these peptides to non-toxic aggregates. These interventions preserve cognitive function in disease-model mice [131]. Cyanidin-3-O-glucopyranoside anthocyanins [132] and malvidin (Figure 1B) [131] directly interfere with Aβ peptide oligomerization into toxic fibrils. These effects may be tied to microglia activation. Blueberry anthocyanins enhance microglial Aβ peptide clearance, inhibiting aggregation via the mitogen-activated protein kinases (MAPKs) pathway [133].
An enhanced antioxidant response is also elicited by anthocyanins. In a model of sporadic Alzheimer’s dementia induced by streptozotocin, commercial anthocyanins from grape skins decreased lipid peroxidation. They restored the level of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), in the cortex and hippocampus [134]. Anthocyanins from black soybean (Glycine max (L.) Merr.) also exhibit direct protection against neuronal cytotoxicity induced by bA injected in the hippocampus [135]. Black soybean anthocyanins enhance HT22 neuron cell viability compared to Aβ-treated cells [135], and blueberry anthocyanins prevent ROS formation and cognitive decline [127]. Natural anthocyanins from Korean black beans reduce ROS levels in mice with high Aβ production and HT22 cells exposed to Aβ oligomers [113,136]. In summary, anthocyanins present promise for AD treatment and prevention, potentially supplementing current therapies due to their established safety.
Finally, the effect of anthocyanins on AD is associated with the regulation of cholinergic neurotransmission by AChE inhibition. In vivo studies showed that the administration of anthocyanins protects against the increase of AChE in the cortex hippocampus [134,137] and cerebellum [138] in models of cognitive deficits associated with AD. In addition, administration of anthocyanin-rich blueberry extract (Vaccinium angustifolium) to mice decreases AChE activity [139]. The inhibition was significantly higher in the brain compared to other tissues [139], suggesting a preferential biodistribution or a selective binding to the AChE isoform expressed in the brain. Treatment of mice with spatial memory impairment with delphidin (50 mg/Kg) reduced AChE activity and amyloid plaque formation in the brain [140].
3. Parkinson’s Disease (PD)
Epidemiological evidence suggests that consumption of anthocyanin-rich berries, like blueberries or strawberries, may mitigate the risk of PD [141]. Clinical studies indicate that blackcurrant (Ribes nigrum) anthocyanins augment the neuroprotective cyclic glycine-proline concentration in Parkinson’s patients’ cerebrospinal fluid [142]. An anthocyanin-rich blackberry extract showed preventive effects against bradykinesia and dopaminergic neuronal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a PD model [143].
In PD, dopaminergic cell death involves mitochondrial complex I impairment, oxidative stress, microglial activation, and Lewy body formation. Blueberry and grape seed extracts restored mitochondrial respiration defects caused by rotenone exposure in dopaminergic cell lines, suggesting improved mitochondrial function and potential neurodegeneration alleviation [144]. Anthocyanins from grape (Vitis vinifera) and Japanese knotweed (Polygonum cuspidatum) enhanced climbing ability in a transgenic Drosophila PD model expressing human alpha-synuclein [145]. Pelargonidin (Figure 1B) anthocyanin exhibited neuroprotective effects against 6-OHDA-induced toxicity by reducing oxidative stress [146]. Anthocyanins from mulberry (Morus alba L.) fruit protected dopaminergic neurons against MPTP exposure by regulating ROS and NO generation, reducing Bcl-2 and Bax expression, mitochondrial membrane depolarization, and caspase-3 activation [143].
4. Hypoxia/Cerebral Ischemia
Ischemic stroke is due to a transient or permanent reduction in cerebral blood flow. The main mechanisms of ischemia/reperfusion injury include excitotoxicity, oxidative stress, inflammation, and apoptosis [147]. In murine models of cerebral ischemia/reperfusion injury, treatment with commercial anthocyanins [148], anthocyanins obtained from the dried fruits of Lycium ruthenicum Murr. [149], or purified anthocyanins from Myrica rubra [150] protect against middle cerebral artery occlusion injury, altering apoptosis and inflammation. Similarly, cyanidin 3-O-β-glucopyranoside has a neuroprotective effect in an animal cerebral artery occlusion model [151]. In mice under conditions of transient global ischemia, anthocyanins from black rice (Oryza sativa L., Poaceae) attenuate neuronal cell death, inhibit reactive astrogliosis, and prevent loss of expression of glutathione peroxidase in the hippocampus, significantly improving memory impairment [152].
As for other diseases discussed above, the amelioration of neuronal injury by anthocyanins in ischemia/reperfusion can be partially explained by their anti-apoptotic, anti-inflammatory, and anti-oxidative activities. Anthocyanins reduce neuronal apoptosis induced by ischemia and/or reperfusion through regulation of the expression of Bcl-2 family proteins [149], reduction of cytochrome c and caspase-3 [153,154,155], and suppression of JNK/p53 pathway [156]. Purified extracts of Myrica rubra anthocyanins protect against cerebral ischemia-reperfusion injury by modulating the TLR4/NF-κB, NLRP3, and Nrf2 signaling pathways [150] and by reducing the levels of inflammatory molecules, including TNF-α, IL-1β, and IL-6 [149]. In neuron cultures, anthocyanins from black soybean (Glycine max (L.) or purified cyanidin-3-glucoside protect from the cytotoxicity induced by oxygen–glucose deprivation by inhibiting oxidative stress and preserving the mitochondrial membrane potential [157]. The effects and mechanism of anthocyanins described here make them candidates for consideration as a dietary supplement to reduce ischemia injury.
This entry is adapted from the peer-reviewed paper 10.3390/biom13111598
This entry is offline, you can click here to edit this entry!
