Among the human T-lymphotropic virus (HTLV) types, HTLV-1 is the most prevalent, and it has been linked to a spectrum of diseases, including HAM/TSP, ATLL, and hyperinfection syndrome or disseminated strongyloidiasis. There is no globally standard first-line treatment for HTLV-1 infection and its related diseases. To address this, a comprehensive research was conducted, analyzing 30 recent papers from databases PubMed, CAPES journals, and the Virtual Health Library (VHL). The studies encompassed a wide range of therapeutic approaches, including antiretrovirals, immunomodulators, antineoplastics, amino acids, antiparasitics, and even natural products and plant extracts. Notably, the category with the highest number of articles was related to drugs for the treatment of ATLL. Studies employing mogamulizumab as a new perspective for ATLL received greater attention in the last 5 years, demonstrating efficacy, safe use in the elderly, significant antitumor activity, and increased survival time for refractory patients. Concerning HAM/TSP, despite corticosteroid being recommended, a more randomized clinical trial is needed to support treatment other than corticoids. The research also included a comprehensive review of the drugs used to treat disseminated strongyloidiasis in co-infection with HTLV-1, including their administration form, in order to emphasize gaps and facilitate the development of other studies aiming at better-directed methodologies. Additionally, docking molecules and computer simulations show promise in identifying novel therapeutic targets and repurposing existing drugs. These advances are crucial in developing more effective and targeted treatments against HTLV-1 and its related diseases.
- HTLV-1-related diseases
- drug treatment
- ATLL
- HAM/TSP
- molecular docking
1. Introduction
2. New Studies about Drugs to Treat HTLV-1 Infection
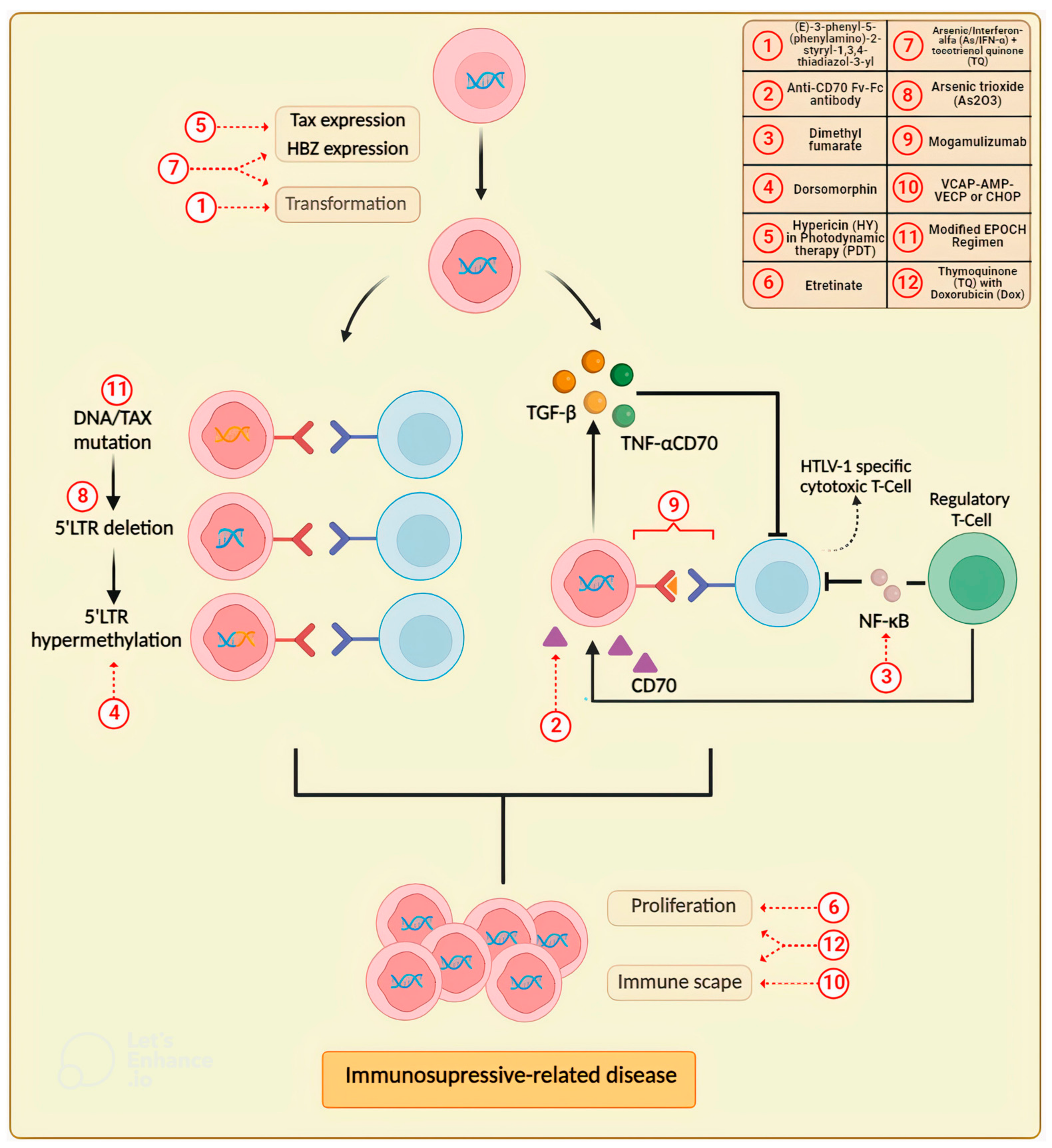
3. Updates in Drug Research for the Treatment of Adult T-Cell Leukemia/Lymphoma (ATLL)
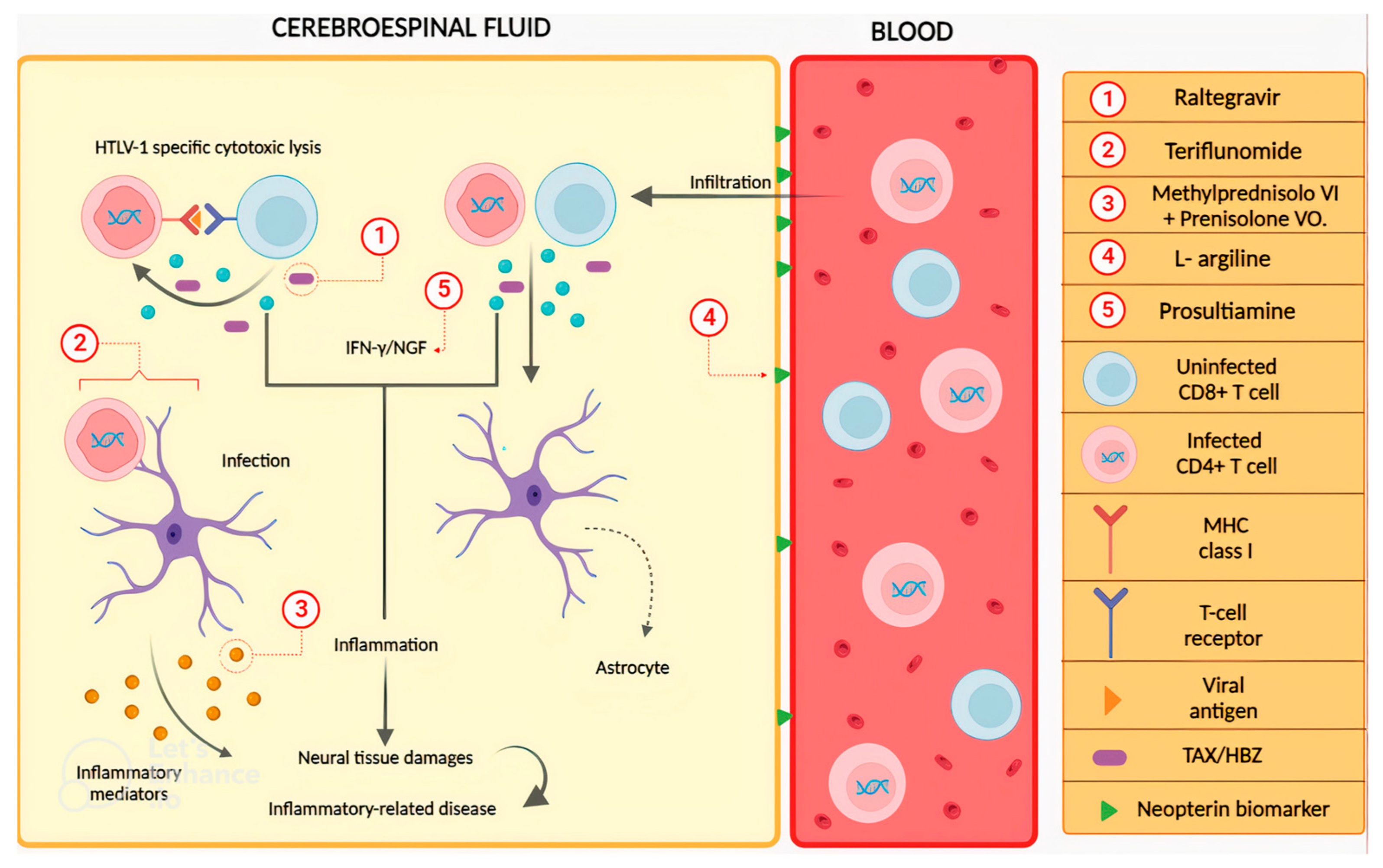
4. Drugs to Treat HTLV-1-Associated Myelopathy (HAM)/Tropical Spastic Paraparesis (TSP)
5. Molecular Docking as a Tool to Discover New Drugs against HTLV-1 Infection and Related Diseases
This entry is adapted from the peer-reviewed paper 10.3390/ph16111546
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type-c retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous t-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419.
- Ministério da Saúde. Guia de Manejo Clínico do Paciente com HTLV; Ministério da Saúde: Rio de Janeiro, Brazil, 2003.
- Martinez, M.P.; Al-Saleem, J.; Green, P.L. Comparative virology of HTLV-1 and HTLV-2. Retrovirology 2019, 16, 21.
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068.
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388.
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30.
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Prosser, A.T.; Lebreton, M.; Mpoudi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Nat. Acad. Sci. USA 2005, 102, 7994–7999.
- Calattini, S.; Betsem, E.; Bassot, S.; Chevalier, S.A.; Mahieux, R.; Froment, A.; Gessain, A. New strain of human T lymphotropic virus (HTLV) type 3 in a Pygmy from Cameroon with peculiar HTLV serologic results. J. Infect. Dis. 2009, 199, 561–564.
- Zheng, H.; Wolfe, N.D.; Sintasath, D.M.; Tamoufe, U.; Lebreton, M.; Djoko, C.F.; Diffo Jle, D.; Pike, B.L.; Heneine, W.; Switzer, W.M. Emergence of a novel and highly divergent HTLV-3 in a primate hunter in Cameroon. Virology 2010, 401, 137–145.
- Hoshino, H. Cellular Factors Involved in HTLV-1 Entry and Pathogenicit. Front Microbiol. 2012, 21, 222.
- Tezuka, K.; Fuchi, N.; Okuma, K.; Tsukiyama, T.; Miura, S.; Hasegawa, Y.; Nagata, A.; Komatsu, N.; Hasegawa, H.; Sasaki, D. HTLV-1 targets human placental trophoblasts in seropositive pregnant women. J. Clin. Investig. 2020, 130, 6171–6186.
- Eusebio-Ponce, E.; Anguita, E.; Paulino-Ramirez, R.; Candel, F.J. HTLV-1 infection: An emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Esp. Quimioter. 2019, 32, 485–496.
- Koyanagi, Y.; Itoyama, Y.; Nakamura, M.; Takamatsu, K.; Kira, J.; Iwamasa, T.; Goto, I.; Yamamoto, N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 1993, 196, 25–33.
- World Health Organization. Human T-Lymphotropic Virus Type 1; Technical Report; World Health Organization: Geneva, Italy, 2021.
- Van-Leeuwen, R.; Katlam, C.; Kitchen, V.; Boucher, C.A.; Tubiana, R.; McBride, M.; Ingrand, D.; Weber, J.; Hill, A.; McDade, H.; et al. Evaluation of safety and efficacy of 3TC (lamivudine) in patients with asymptomatic or mildly symptomatic human immunodeficiency virus infection: A phase I/II study. J. Infect. Dis. 1995, 171, 1166–1171.
- Taylor, G.P.; Hall, S.E.; Navarrete, S.; Michie, C.A.; Davis, R.; Witkover, A.D.; Rossor, M.; Nowak, M.A.; Rudge, P.; Matutes, E.; et al. Effect of Lamivudine on Human T-Cell Leukemia Virus Type 1 (HTLV-1) DNA Copy Number, T-Cell Phenotype, and Anti-Tax Cytotoxic T-Cell Frequency in Patients with HTLV-1-Associated Myelopathy. J. Virol. 1999, 73, 10289–10295.
- Iwanaga, M. Epidemiology of HTLV-1 Infection and ATL in Japan: An Update. Front. Microbiol. 2020, 11, 1124.
- Cook, L.B.; Fuji, S.; Hermine, O.; Bazarbachi, A.; Ramos, J.C.; Ratner, L.; Horwitz, S.; Fields, P.; Tanase, A.; Bumbea, H.; et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 2019, 37, 677–687.
- Araujo, A.; Bangham, C.R.M.; Casseb, J.; Gotuzzo, E.; Jacobson, S.; Martin, F.; Penalva de Oliveira, A.; Puccioni-Sohler, M.; Taylor, G.P.; Yamano, Y. Management of HAM/TSP: Systematic Review and Consensus-based Recommendations 2019. Neurol. Clin. Pract. 2021, 11, 49–56.
- Boxus, M.; Willems, L. Mechanisms of HTLV-1 persistence and transformation. Br. J. Cancer 2009, 101, 1497–1501.
- Santos, D.F.; De Pilger, D.R.B.; Vandermeulen, C.; Khouri, R.; Mantoani, S.P.; Nunes, P.S.G.; De andrade, P.; Carvalho, I.; Casseb, J.; Twizere, J.C.; et al. Non-cytotoxic 1,2,3-triazole tethered fused heterocyclic ring derivatives display Tax protein inhibition and impair HTLV-1 infected cells. Bioorg. Med. Chem. 2020, 28, 115746.
- Sousa-Pereira, D.; Oliveira, T.S.; Paiva, R.O.; Chaves, O.A.; Netto-Ferreira, J.C.; Echavarria-lima, J.; Echevarria, A. Synthetic (E)-3-Phenyl-5-(phenylamino)-2-styryl-1,3,4-thiadiazol-3-ium Chloride Derivatives as Promising Chemotherapy Agents on Cell Lines Infected with HTLV-1. Molecules 2020, 25, 2537.
- Gutowska, A.; Mckinnon, K.; Sarkis, S.; Doster, M.N.; Bissa, M.; Moles, R.; Stamos, J.D.; Rahman, M.A.; Washington-Parks, R.; Davis, D.; et al. Transient Viral Activation in Human T Cell Leukemia Virus Type 1-Infected Macaques Treated with Pomalidomide. Front. Med. 2022, 9, 897264.
- Karbalaei, M.; Keikha, M. Curcumin as an Herbal Inhibitor Candidate Against HTLV-1 Protease. Jentashapir J. Cell. Mol. Biol. 2019, 10, e92813.
- Nakama, S.; Ishikawa, C.; Nakachi, S.; Mori, N. Anti-adult T-cell leukemia effects of Bidens pilosa. Int. J. Oncol. 2011, 38, 1163–1173.
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337.
- Abu-Jafar, A.; Suleiman, M.; Nesim, N.; Huleihel, M. The effect of alcoholic extract from Eucalyptus camaldulensis leaves on HTLV-1 Tax activities. Cell Cycle 2020, 19, 1768–1776.
- Mulherkar, R.; Karabudak, A.; Ginwala, R.; Huang, X.; Rowan, A.; Philip, R.; Murphy, E.L.; Clements, D.; Ndhlovu, L.C.; Khan, Z.K.; et al. In vivo and in vitro immunogenicity of novel MHC class I presented epitopes to confer protective immunity against chronic HTLV-1 infection. Vaccine 2019, 36, 5046–5057.
- Soltani, A.; Hashemy, S.I.; Zahedi, A.F.; Soleimani, A.; Rafatpanah, H.; Rezaee, S.A.; Griffith, R.; Mashkani, B. Molecular targeting for treatment of human T-lymphotropic virus type 1 infection. Biomed. Pharmacother. 2019, 109, 770–778.
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580.
- Utsunomiya, A.; Choi, I.; Chihara, D.; Seto, M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015, 106, 344–351.
- Kinpara, S.; Kijiyama, M.; Takamori, A.; Hasegawa, A.; Sasada, A.; Masuda, T.; Tanaka, Y.; Utsunomiya, A.; Kannagi, M. Interferon-a (IFN-a) suppresses HTLV-1 gene expression and cell cycling, while IFN-acombined with zido-vudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 2013, 10, 52.
- Tsukamoto, Y.; Kiyasu, J.; Choi, I.; Kozuru, M.; Uike, N.; Utsunomiya, H.; Hirata, A.; Fujioka, E.; Ohno, H.; Nakashima, E.; et al. Efficacy and Safety of the Modified EPOCH Regimen (Etoposide, Vincristine, Doxorubicin, Carboplatin, and Prednisolone) for Adult T-cell Leukemia/Lymphoma: A Multicenter Retrospective Study. Clin. Lymphoma Myeloma Leuk. 2020, 20, e445–e453.
- Fuji, S.; Yamaguchi, T.; Inoue, Y.; Utsunomiya, A.; Moriuchi, Y.; Owatari, S.; Miyagi, T.; Sawayama, Y.; Otsuka, E.; Yoshida, S.I.; et al. VCAP-AMP-VECP as a preferable induction chemotherapy in transplant-eligible patients with aggressive adult T-cell leukemia-lymphoma: A propensity score analysis. Bone Marrow Transpl. 2019, 54, 1399–1405.
- Ishida, T.; Utsunomiya, A.; Iida, S.; Inagaki, H.; Takatsuka, Y.; Kusumoto, S.; Takeuchi, G.; Shimizu, S.; Ito, M.; Komatsu, H.; et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003, 9, 3625–3634.
- Phillips, A.A.; Fields, P.A.; Hermine, O.; Ramos, J.C.; Beltran, B.E.; Pereira, J.; Wandroo, F.; Feldman, T.; Taylor, G.P.; Sawas, A.; et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 2019, 104, 993–1003.
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842.
- Satake, A.; Konishi, A.; Azuma, Y.; Tsubokura, Y.; Yoshimura, H.; Hotta, M.; Nakanishi, T.; Fujita, S.; Nakaya, A.; Ito, T.; et al. Clinical efficacy of mogamulizumab for relapsed/refractory aggressive adult T-cell leukemia/lymphoma: A retrospective analysis. Eur. J. Haematol. 2020, 105, 704–711.
- Ishitsuka, K.; Yurimoto, S.; Tsuji, Y.; Iwabuchi, M.; Takahashi, T.; Tobinai, K. Safety and effectiveness of mogamulizumab in relapsed or refractory adult T-cell leukemia-lymphoma. Eur. J. Haematol. 2019, 102, 407–415.
- Yonekura, K.; Takeda, K.; Kawakami, N.; Kanzaki, T.; Kanekura, T.; Utsunomiya, A. Therapeutic Efficacy of Etretinate on Cutaneous-type Adult T-cell Leukemia-Lymphoma. Acta Derm Venereol. 2019, 99, 774–776.
- Aikawa, A.; Kozako, T.; Uchida, Y.; Yoshimitsu, M.; Ishitsuka, K.; Ohsugi, T.; Honda, S.I. Cell death induced by dorsomorphin in adult T-cell leukemia/lymphoma is AMPK-independent. FEBS J. 2020, 287, 4005–4015.
- Schmitt, A.; Xu, W.; Bucher, P.; Grimm, M.; Konantz, M.; Horn, H.; Zapukhlyak, M.; Berning, P.; Brändle, M.; Jarboui, M.A.; et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 2021, 138, 871–884.
- Maeta, T.; Sato, T.; Asano, K.; Ito, S. Dimethyl Fumarate Induces Apoptosis via Inhibiting NF-κB and STAT3 Signaling in Adult T-cell Leukemia/Lymphoma Cells. Anticancer Res. 2022, 42, 2301–2309.
- Sato, T.; Maeta, T.; Ito, S. Dimethyl Fumarate Suppresses the Proliferation of HTLV-1-infected T Cells by Inhibiting CBM Complex-triggered NF-B Signaling. Anticancer Res. 2023, 43, 1901–1908.
- Yokota, R.; Hashimoto, S.; Watanabe, I.; Kishimoto, S.; Toyama, M.; Okamoto, M.; Yoshimtsu, M.; Ishitsuka, K.; Ito, I.; Baba, M. Novel Anti-CD70 Antibody Drug Conjugate for the Treatment of Adult T-Cell Leukemia (ATL). Anticancer Res. 2020, 40, 4471–4479.
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5.
- Fatfat, M.; Fakhoury, I.; Habli, Z.; Mismar, R.; Gali-Muhtasib, H. Thymoquinone enhances the anticancer activity of doxorubicin against adult T-cell leukemia in vitro and in vivo through ROS-dependent mechanisms. Life Sci. 2019, 232, 116628.
- Houssein, M.; Fatfat, M.; Habli, Z.; Ghazal, N.; Moodad, S.; Khalife, H.; Khalil, M.; Gali-Muhtasib, H. Thymoquinone synergizes with arsenic and interferon alpha to target human T-cell leukemia/lymphoma. Life Sci. 2020, 251, 117639.
- Marçais, A.; Cook, L.; Witkover, A.; Asnafi, V.; Avettand-Fenoel, V.; Delarue, R.; Cheminant, M.; Sibon, D.; Frenzel, L.; De Thé, H.; et al. Arsenic trioxide (As2O3) as a maintenance therapy for adult T cell leukemia/lymphoma. Retrovirology 2020, 17, 5.
- Futsch, N.; Mahieux, R.; Dutartre, H. HTLV-1, the other pathogenic yet neglected human retrovirus: From transmission to therapeutic treatment. Viruses 2017, 10, 1.
- Rajaei, T.; Farajifard, H.; Rezaee, S.A.; Azarpazhooh, M.R.; Mahmoudi, M.; Valizadeh, N.; Rafatpanah, H. Different roles of CXCR1 and CXCR2 in HTLV-1 carriers and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. Med. Microbiol. Immunol. 2019, 208, 641–650.
- Nozuma, S.; Jacobson, S. Neuroimmunology of Human T-Lymphotropic Virus Type 1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2019, 10, 885.
- Nakamura, T. HAM/TSP Pathogenesis: The Transmigration Activity of HTLV-1-Infected T Cells into Tissues. Pathogens 2023, 12, 492.
- Yamauchi, J.; Tanabe, K.; Sato, T.; Nakagawa, M.; Matsuura, E.; Tsuboi, Y.; Tamaki, K.; Sakima, H.; Ishihara, S.; Ohta, Y.; et al. Efficacy of Costicosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P). Viruses 2022, 14, 136.
- Enose-Akahata, Y.; Billioux, B.J.; Azodi, S.; Dwyer, J.; Velluci, A.; Ngouth, N.; Nozuma, S.; Massoud, R.; Cortese, I.; Ohayon, N.; et al. Clinical trial of raltegravir, an integrase inhibitor, in HAM/TSP. Ann. Clin. Transl. Neurol. 2021, 8, 1970–1985.
- Enose-Akahata, Y.; Ngouth, N.; Ohayon, J.; Mandel, M.; Chavin, J.; Turner, T.J.; Jacobson, S. Effect of Teriflunomide on cells from patients with Human T-cell Lymphotropic Virus type 1-associated neurological disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e986.
- Matsuo, T.; Miyata, Y.; Nakamura, T.; Satoh, K.; Sakai, H. Prosultiamine for treatment of lower urinary tract dysfunction accompanied by human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. Int. J. Urol. 2018, 25, 54–60.
- Nozuma, S.; Matsuura, E.; Tashiro, Y.; Nagata, R.; Ando, M.; Hiramatsu, Y.; Higuchi, Y.; Sakiyama, Y.; Hashiguchi, A.; Michizono, K.; et al. Efficacy of l-Arginine treatment in patients with HTLV-1-associated neurological disease. Ann. Clin. Transl. Neurol. 2023, 10, 237–245.
- Park, K.B.; Dalton-Brown, E.; Hirst, C.; Williams, D.P. Selection of new chemical entities with decreased potential for adverse drug reactions. Eur. J. Pharmacol. 2006, 549, 1–8.
- Jakhar, R.; Hooda, M.D.; Khici, A.; Chhillar, A. Relevance of molecular docking studies in drug designing. Curr. Bioinform. 2020, 15, 270–278.
- Hoces, D.; Barros, N.; Woll, F.; Bauer, A.; White, A.C., Jr.; Montes, M. Regulatory T cell expansion resolves after effective strongyloidiasis treatment in subjects with HTLV-1 co- infection. Parasitol. Int. 2020, 76, 102092.
- Marino-Merlo, F.; Balestrieri, E.; Matteucci, C.; Mastino, A.; Grelli, S.; Macchi, B. Antiretroviral Therapy in HTLV-1 Infection: An Updated Overview. Pathogens 2020, 9, 342.
- Kassay, N.; Motyan, J.A.; Matuz, K.; Golda, M.; Tozser, J. Biochemical characterization, specificity and inhibition studies of HTLV-1, HTLV-2, and HTLV-3 proteases. Life 2021, 11, 127.
- Jahantigh, H.; Ahmadi, N.; Lovreglio, P.; Stufano, A.; Enayatkhani, M.; Shahbazi, B.; Ahmadi, K. Repurposing antiviral drugs against HTLV-1 protease by molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2023, 41, 5057–5066.
- Selvaraj, C.; Singh, P.; Singh, S.K. Molecular modeling studies and comparative analysis on structurally similar HTLV and HIV protease using HIV-PR inhibitors. J. Recept. Signal Transduct. Res. 2014, 34, 361–371.
- Taylor, K.; Das, S.; Pearson, M.; Kozubek, J.; Strivens, M.; Gardner, S. Systematic drug repurposing to enable precision medicine: A case study in breast cancer. Digital Medicine. 2019, 5, 180–186.
- Sohraby, F.; Aryapour, H. Reconstruction of the binding pathway of an anti-HIV drug, Indinavir, in complex with the HTLV-1 protease using unaggregated unbiased molecular dynamics simulation. Comput. Biol. Chem. 2022, 96, 107616.
