Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pain is a complex entity with deleterious effects on the entire organism. Poorly controlled postoperative pain impacts the patient outcome, being associated with increased morbidity, inadequate quality of life and functional recovery.
- pain pathways
- colorectal surgery
- multimodal analgesia
1. Introduction
According to the International Association for the Study of Pain (IASP), pain is ‘An unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.’ Awareness of the high intensity of intraoperative surgical pain led to the birth of a new medical specialty: anesthesiology (from Ancient Greek roots ἀν- an—‘not’, αἴσθησις- aísthēsis-‘—‘sensation’, and ‘λογία- logia’—‘study’). Underestimation of acute postoperative pain contributes to chronic pain development with a permanently poor quality of life [1]. Surgical pain induces a profound physiologic neurohumoral response, the so-called “adaptive stress response”, which sometimes might exceed its protective role and becomes harmful. Choosing the anesthetic technique and perioperative analgesia equally implies taking into consideration patient factors and surgical procedure stress. Minimally invasive surgical techniques are increasingly utilized, altering analgesic requirements compared with open laparotomy [2].
2. Acute Pain after Surgery Pathways
Cortical representation of a pain stimulus implies a four-stage process (see Figure 1):
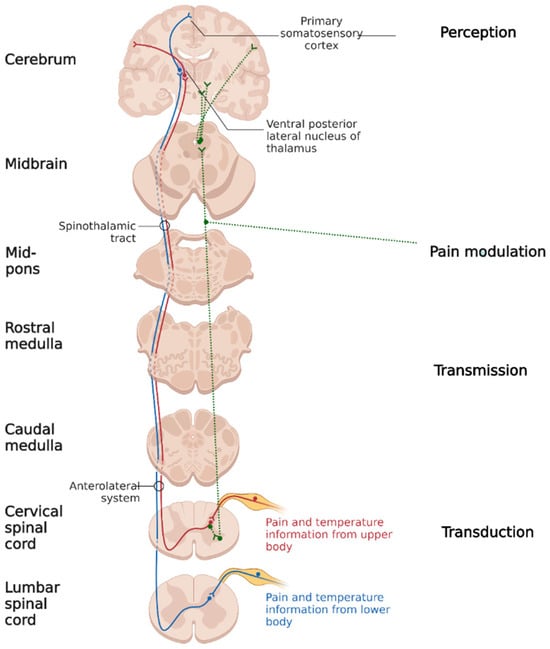
Figure 1. Discriminative pain pathways (created using BioRender.com).
1. The first stage is transduction, meaning a stimulus’ conversion into action potential. Surgical trauma activates nociceptors through their direct mechanical, electrical or thermal stimulation, as well as through cellular destruction, with the secondary release of adenosine, protons, potassium, and bradykinin, which chemically activates the polymodal nociceptors. The antidromic substance P and glutamate released from the afferents of the nociceptive nerve fibers lead to the augmentation of inflammation. All these mechanisms contribute to peripheral nociceptor sensitization, causing allodynia (pain from nonpainful stimuli) and primary hyperalgesia (exaggerated pain from a painful stimulus) [3][4][5].
2. The second step is transmission, which is the conduction of the action potential through A δ (somatic parietal pain) and C (visceral pain) nerve fibers. The synapse between primary and secondary neurons takes place in the dorsal horn of the spinal cord. At the synaptic level, the main mediator involved in pain transmission is glutamate, which stimulates receptors coupled with AMPA sodium channels [5]. Repetitively stimulating the secondary neurons increases the membrane density of AMPA receptors. Iterative stimulation leads to the expansion of the nervous afferent territory. Thus, secondary hyperalgesia occurs (amplification of the painful perception of stimulus coming from inside and outside the injured area) through the phenomenon of central sensitization [6][7].
Secondary neuron axons form the ascending tracts: spinothalamic, spinoreticular and spinohypotalamic connected to the hypothalamus, brainstem, limbic system, ascending activating reticulated system and periaqueductal gray substance. These connections take part in the neuroendocrine and vegetative pain response and give the affective/emotional characteristic to surgical pain.
3. The third stage is pain modulation. At present, two theories might explain the modulation phenomenon: the first one is based on the descending modulatory system activity (starting from the midbrain periaqueductal gray substance and receiving input from the hypothalamus, thalamus, limbic system, and cortex)—this system is activated by endogenous and exogenous opioids and makes synapses with the inhibitory interneurons from the dorsal horn of the spinal cord. The second one is gate-control theory: a mechanism in the spinal cord in which pain signals go up to the brain and are processed to accentuate or attenuate the possible perceived pain at the spinal cord itself.
4. The fourth stage is pain perception. The primary and secondary somatosensory cortex (pain location and duration), limbic system (affective/emotional pain component) and prefrontal cortex (anticipating pain by modeling past experiences) have been identified as regions associated with pain perception [8].
3. Pain and Surgical Stress Response
Pain is the main component of surgical stress, leading to the activation of the hypothalamic–pituitary–adrenal axis, with the subsequent increase in serum levels of cortisol, growth hormone and vasopressin, and to sympathetic nervous system activation, all components of the systemic stress response [9]. Cortisol is released very soon after the beginning of surgery with a peak secretion at 4–6 h and, depending on the extent of the surgery, can reach values up to four times higher than the basal ones [10]. High cortisol and glucagon serum levels and decreased insulin secretion caused by sympathetic stimulation lead to catabolic syndrome. Persistent hypercatabolism may lead to increased hospitalization and mortality. Hyperglycemia also increases the infection risk.
Untreated pain causes prolonged sympathetic stimulation which, in association with anemia and perioperative hypoxia, exposes patients with cardiac risk factors to postoperative myocardial injury/infarction (PMI) [11][12]. Data from a large retrospective cohort analysis published in 2020 by Turan et al. concluded that, among patients undergoing noncardiac surgery, high time-weighted average pain scores within 72 h after surgery were significantly associated with greater risk of myocardial injury compared to lower pain scores [13].
Liberal perioperative crystalloid infusion and vasopressin release effects through renin–angiotensin–aldosterone system activation produce interstitial space expansion and postoperative edema. Hypoalbuminemia usually happens due to albumin distribution volume augmentation and increased capillary permeability with secondary escape of serum albumin into the interstitial space. The main consequence is interstitial edema, including at the bowel level, one of the causes of postoperative ileus and postoperative gastrointestinal tract dysfunction with an elevated risk of anastomotic leakage. In addition, manipulation of the bowel, increased sympathetic stimulation, opioid use and fasting are also contributors to the inhibition of gastrointestinal peristalsis [14]. Increased perioperative levels of catecholamine may be associated with pro-metastatic effects [15].
Higher levels of postoperative pain and pain distress can increase morbidity, prevent functional recovery, and reduce the quality of life. Furthermore, suboptimal postoperative analgesia is a risk factor for ongoing opioid use, opioid dependence and persistent post-surgical pain [16].
4. Methods to Manage Surgical Pain
The surgical stress response including its physiological derangements becomes a challenge to postoperative patients. The use of standardized enhanced recovery programs in combination with laparoscopic surgery has revolutionized clinical and patient-reported outcomes in elective colorectal surgery. Central to the success of enhanced recovery programs is optimal pain management [17]. Minimally invasive surgical techniques are increasingly utilized, altering analgesic requirements compared with open laparotomy, leading to a decline in the popularity of epidural anesthesia and increasing interest in intrathecal morphine and truncal nerve blocks [2]. There is a paucity of data identifying the ideal analgesic regimen in laparoscopic colorectal surgery and a lack of consensus on the optimal perioperative analgesia strategy in this cohort of patients [17].
The number of hospitalized patients with pre-existing opioid tolerance, chronic pain, or opioid use disorder (OUD) is also increasing, further challenging prescribers and straining healthcare resources [18]. These patients need customized care plans for perioperative analgesia.
Pain management in colorectal surgery requires multimodal analgesia utilizing complementary mechanisms to improve pain control with less high-risk drug exposure. This concept is based on pain prevention and remission through blocking nerve impulse transmission at different stations over the nervous system.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12216771
References
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.M.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013, 118, 934–944.
- Pirie, K.; Traer, E.; Finniss, D.; Myles, P.S.; Riedel, B. Current approaches to acute postoperative pain management after major abdominal surgery: A narrative review and future directions. Br. J. Anaesth. 2022, 129, 378–393.
- Carreira, E.U.; Carregaro, V.; Teixeira, M.M.; Moriconi, A.; Aramini, A.; Verri, W.A.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur. J. Pain 2013, 17, 654–663.
- Kim, T.J.; Freml, L.; Park, S.S.; Brennan, T.J. Lactate Concentrations in Incisions Indicate Ischemic-like Conditions May Contribute to Postoperative Pain. J. Pain 2007, 8, 59–66.
- Paul, R.O.; Barash, G.; Cullen, B.F.; Stoelting, R.K.; Cahalan, M.K.; Christine Stock, M. Acute pain management. In Clinical Anesthesia; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1614–1616.
- Marchand, S. The Physiology of Pain Mechanisms: From the Periphery to the Brain. Rheum. Dis. Clin. N. Am. 2008, 34, 285–309.
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain—From mechanisms to treatment. Pain Rep. 2017, 2, e588.
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511.
- Finnerty, C.C.; Mabvuure, N.T.; Kozar, R.A.; Herndon, D.N. The Surgically Induced Stress Response. J. Parenter. Enter. Nutr. 2013, 37, 21S.
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117.
- Puelacher, C.; Lurati-Buse, G.; Singeisen, H.; Dang, M.; Cuculi, F.; Mueller, C. Perioperative myocardial infarction/injury after noncardiac surgery. Swiss Med. Wkly. 2015, 145, w14219.
- Botto, F.; Alonso-Coello, P.; Chan, M.T.V.; Villar, J.C.; Xavier, D.; Srinathan, S.; Guyatt, G.; Cruz, P.; Graham, M.; Wang, C.Y.; et al. Myocardial Injury after Noncardiac Surgery: A Large, International, Prospective Cohort Study Establishing Diagnostic Criteria, Characteristics, Predictors, and 30-day Outcomes. Anesthesiology 2014, 120, 564–578.
- Turan, A.; Leung, S.; Bajracharya, G.R.; Babazade, R.; Barnes, T.; Schacham, Y.N.; Mao, G.; Zimmerman, N.; Ruetzler, K.; Maheshwari, K.; et al. Acute Postoperative Pain Is Associated with Myocardial Injury after Noncardiac Surgery. Anesth. Analg. 2020, 131, 822–829. Available online: https://journals.lww.com/anesthesia-analgesia/fulltext/2020/09000/acute_postoperative_pain_is_associated_with.24.aspx (accessed on 23 October 2023).
- Bragg, D.; El-Sharkawy, A.M.; Psaltis, E.; Maxwell-Armstrong, C.A.; Lobo, D.N. Postoperative ileus: Recent developments in pathophysiology and management. Clin. Nutr. 2015, 34, 367–376.
- Haldar, R.; Ben-Eliyahu, S. Reducing the risk of post-surgical cancer recurrence: A perioperative anti-inflammatory anti-stress approach. Future Oncol. 2018, 14, 1017–1021.
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298.
- Brown, L.; Gray, M.; Griffiths, B.; Jones, M.; Madhavan, A.; Naru, K.; Shaban, F.; Somnath, S.; Harji, D. A multicentre, prospective, observational cohort study of variation in practice in perioperative analgesia strategies in elective laparoscopic colorectal surgery (the LapCoGesic study). Ann. R. Coll. Surg. Engl. 2020, 102, 28–35.
- Hyland, S.J.; Wetshtein, A.M.; Grable, S.J.; Jackson, M.P. Acute Pain Management Pearls: A Focused Review for the Hospital Clinician. Healthcare 2023, 11, 34.
This entry is offline, you can click here to edit this entry!
