Sustainable agriculture requires factors to directly stimulate plant growth and induce the plant’s innate immune system to protect against stresses. Protection of plants is one of the main approaches to the supply of food resource. Furthermore, improved techniques for plant disease management must be environmentally sustainable, reliable, acceptable by society, and chemical-free to ensure sustainable food security. Although it is not possible to accurately determine postharvest losses due to diseases and physiological disorders, the use of proper harvesting and transportation methods that minimize damage to the product, along with optimal storage conditions that prevent the development of diseases, will be effective in reducing these postharvest losses. Since handling and storage conditions are potential threats for postharvest spoilage, it is necessary to identify environmentally friendly approaches and their precision mechanisms for postharvest disease management. Recently, biological control, non-chemical, and eco-friendly techniques have been investigated for this purpose.
- biological control
- biosensors
- combined treatments
- edible coatings
- nanotechnology
1. Introduction
2. Postharvest Diseases Management
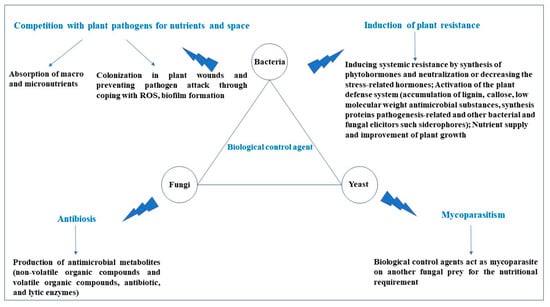
2.1. Biological Control
2.2. Biosensors
2.3. Nanotechnology
2.4. Plant Growth Regulators (PGRs)
2.5. Edible Coatings
2.6. Essential Oils (EOs)
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9101099
References
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Harding, R. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1496.
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16.
- Xu, J.; Xian, Q.; Wang, K.; Dong, J.; Zhang, C.; Du, S.; Chen, X. Screening and identification of candidate Fusarium wilt-resistance genes from pumpkin. Hortic. Plant J. 2022, 8, 583–592.
- Xu, S.; Bai, T.; Zhang, L.; Fan, H.; Yang, P.; Yin, K.; Zheng, S. Evaluation of different banana varieties on Fusarium wilt TR4 resistance by phenotypic symptom and real-time quantitative PCR. Southwest China J. Agric. Sci. 2017, 30, 1997–2002.
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250.
- Rodríguez, A.; San Andrés, V.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Peña, L. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011, 156, 793–802.
- Rodríguez, A.; Shimada, T.; Cervera, M.; Alquézar, B.; Gadea, J.; Gómez-Cadenas, A.; Peña, L. Terpene down-regulation triggers defense responses in transgenic orange leading to resistance against fungal pathogens. Plant Physiol. 2014, 164, 321–339.
- Che, J.; Chen, Y.; Wu, Y.; Li, L.; Tao, N. Metabolomics analysis reveals that myrcene stimulates the spore germination of Penicillium digitatum via the upregulation of central carbon and energy metabolism. Postharvest Biol. Technol. 2020, 170, 111329.
- Tao, N.; Chen, Y.; Wu, Y.; Wang, X.; Li, L.; Zhu, A. The terpene limonene induced the green mold of citrus fruit through regulation of reactive oxygen species (ROS) homeostasis in Penicillium digitatum spores. Food Chem. 2019, 277, 414–422.
- Alegbeleye, O.O.; Sant’Ana, A.S. Risks associated with the consumption of irrigation water contaminated produce: On the role of quantitative microbial risk assessment. Curr. Opin. Food Sci. 2021, 41, 88–98.
- Pruvost, O.; Couteau, A.; Luisetti, J. Development of bacterial black spot of mangoes and epiphytic populations of the pathogen (Xanthomonas campestris pv. mangiferaeindicae) under natural conditions in Reunion. Fruits 1990, 45, 125–140.
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513.
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757.
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2021, 5, 37.
- Oufensou, S.; Ul Hassan, Z.; Balmas, V.; Jaoua, S.; Migheli, Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins 2023, 15, 45.
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111.
- Sangchote, S. Botryodiplodia stem end rot of mango and its control. In III International Mango Symposium; Acta Hortic.: Darvin, NT, Australia, 1989; Volume 291, pp. 296–303.
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051.
- Wielogórska, E.; MacDonald, S.; Elliott, C.T. A review of the efficacy of mycotoxin detoxifying agents used in feed in light of changing global environment and legislation. World Mycotoxin J. 2016, 9, 419–433.
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40.
- Papoutsis, K.; Mathioudakis, M.M.; Hasperue, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491.
- Bosch, Y.; Britt, E.; Perren, S.; Naef, A.; Frey, J.E.; Buhlmann, A. Dynamics of the apple fruit microbiome after harvest and implications for fruit quality. Microorganisms 2021, 9, 272.
- Perini, M.A.; Sin, I.N.; Jara, A.M.R.; Lobato, M.E.G.; Civello, P.M.; Martínez, G.A. Hot water treatments performed in the base of the broccoli stem reduce postharvest senescence of broccoli (Brassica oleracea L. Var italic) heads stored at 20 C. LWT-Food Sci. Technol. 2017, 77, 314–322.
- Wu, Z.; Yuan, X.; Li, H.; Liu, F.; Wang, Y.; Li, J.; Wang, Y. Heat acclimation reduces postharvest loss of table grapes during cold storage–Analysis of possible mechanisms involved through a proteomic approach. Postharvest Biol. Technol. 2015, 105, 26–33.
- Wassermann, B.; Kusstatscher, P.; Berg, G. Microbiome response to hot water treatment and potential synergy with biological control on stored apples. Front. Microbiol. 2019, 10, 2502.
- Moreno-Vilet, L.; Hernández-Hernández, H.M.; Villanueva-Rodríguez, S.J. Current status of emerging food processing technologies in Latin America: Novel thermal processing. Innov. Food Sci. Emerg. Technol. 2018, 50, 196–206.
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Cruz, A.G. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154.
- Hirt, H. Healthy soils for healthy plants for healthy humans: How beneficial microbes in the soil, food and gut are interconnected and how agriculture can contribute to human health. EMBO Rep. 2020, 21, 51069.
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298.
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48.
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97.
- Luksa, J.; Vepstaitė-Monstavicė, I.; Apsegaitė, V.; Blazytė-Čereskienė, L.; Staneviciene, R.; Strazdaitė-Žielienė, Ž.; Serviene, E. Fungal microbiota of sea buckthorn berries at two ripening stages and volatile profiling of potential biocontrol yeasts. Microorganisms 2020, 8, 456.
- Romero, J.; Albertos, I.; Díez-Méndez, A.; Poveda, J. Control of postharvest diseases in berries through edible coatings and bacterial probiotics. Sci. Hortic. 2022, 304, 111326.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Schloter, M. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016, 7, 955.
- Madbouly, A.K.; Elyousr, K.A.A.; Ismail, I.M. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biol. Control 2020, 144, 104239.
- Cruz, A.F.; Barka, G.D.; Blum, L.E.B.; Tanaka, T.; Ono, N.; Kanaya, S.; Reineke, A. Evaluation of microbial communities in peels of Brazilian tropical fruits by amplicon sequence analysis. Braz. J. Microbiol. 2019, 50, 739–748.
- Hall, M.E.; Wilcox, W.F. Identification and frequencies of endophytic microbes within healthy grape berries. Am. J. Enol. Vitic. 2019, 70, 212–219.
- Chen, C.; Cao, Z.; Li, J.; Tao, C.; Feng, Y.; Han, Y. A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biol. Control 2020, 148, 104306.
- Fresno, D.H.; Munné-Bosch, S. Differential tissue-specific jasmonic acid, salicylic acid, and abscisic acid dynamics in sweet cherry development and their implications in fruit-microbe interactions. Front. Plant Sci. 2021, 12, 640601.
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, 261–265.
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121.
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034.
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118.
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Yang, X. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397.
- Seifi Kalhor, M.; Aliniaeifard, S.; Seif, M.; Javadi, E.; Bernard, F.; Li, T.; Lastochkina, O. Rhizobacterium Bacillus subtilis reduces toxic effects of high electrical conductivity in soilless culture of lettuce. In International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant; Acta Hortic.: Darvin, NT, Australia, 2017; Volume 1227, pp. 471–478.
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439.
- Solanki, M.K.; Yandigeri, M.S.; Kumar, S.; Singh, R.K.; Srivastava, A.K. Co-inoculation of different antagonists can enhance the biocontrol activity against Rhizoctonia solani in tomato. Antonie Leeuwenhoek 2019, 112, 1633–1644.
- Thangavelu, R.; Gopi, M. Field suppression of Fusarium wilt disease in banana by the combined application of native endophytic and rhizospheric bacterial isolates possessing multiple functions. Phytopathol. Mediterr. 2015, 54, 241–252.
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051.
- Maksimov, I.V.; Veselova, S.V.; Nuzhnaya, T.V.; Sarvarova, E.R.; Khairullin, R.M. Plant growth-promoting bacteria in regulation of plant resistance to stress factors. Russ. J. Plant Physiol. 2015, 62, 715–726.
- Duffy, B.; Schouten, A.; Raaijmakers, J.M. Pathogen self-defense: Mechanisms to counteract microbial antagonism. Annu. Rev. Phytopathol. 2003, 41, 501–538.
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717.
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25.
- Singh, B.; Kaur, N.; Kumar, P.; Hallan, V.; Pati, P.K. Reactive oxygen species generating and scavenging systems play critical role in conferring leaf spot disease resistance in Withania somnifera (L.) Dunal. Ind. Crop. Prod. 2020, 157, 112889.
- Jiang, M.Y.; Wang, Z.R.; Chen, K.W.; Kan, J.Q.; Wang, K.T.; Zalán, Z.S.; Du, M.Y. Inhibition of postharvest gray mold decay and induction of disease resistance by Pseudomonas fluorescens in grapes. Acta Aliment. 2019, 48, 288–296.
- Zhou, Q.; Fu, M.; Xu, M.; Chen, X.; Qiu, J.; Wang, F.; Chen, L. Application of antagonist Bacillus amyloliquefaciens NCPSJ7 against Botrytis cinerea in postharvest Red Globe grapes. Food Sci. Nutr. 2020, 8, 1499–1508.
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mold in postharvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456.
- Lu, Y.; Ma, D.; He, X.; Wang, F.; Wu, J.; Liu, Y.; Deng, J. Bacillus subtilis KLBC BS6 induces resistance and defense-related response against Botrytis cinerea in blueberry fruit. Physiol. Mol. Plant Pathol. 2021, 114, 101599.
- Sen, S.; Sengupta, P.; Molla, J.; Mukherjee, K.; Acharya, K. Management of postharvest green mold decay in common mandarin and Indian gooseberry with Bacillus licheniformis SR-14. J. Appl. Hortic. 2018, 20, 129–135.
- Deng, J.; Kong, S.; Wang, F.; Liu, Y.; Jiao, J.; Lu, Y.; Li, X. Identification of a new Bacillus sonorensis strain KLBC GS-3 as a biocontrol agent for postharvest green mold in grapefruit. Biol. Control 2020, 151, 104393.
- Madhupani, Y.D.S.; Adikaram, N.K.B. Delayed incidence of stem-end rot and enhanced defences in Aureobasidium pullulans-treated avocado (Persea americana Mill.) fruit. J. Plant Dis. Prot. 2017, 124, 227–234.
- Granada, D.; Lopez-Lujan, L.; Ramirez-Restrepo, S.; Morales, J.; Pelaez-Jaramillo, C.; Andrade, G.; Bedoya-pérez, J.C. Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integr. Agric. 2020, 19, 748–758.
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol. Control 2018, 126, 136–141.
- Qiao, Z.; Fu, Y.; Lei, C.; Li, Y. Advances in antimicrobial peptides-based biosensing methods for detection of foodborne pathogens: A review. Food Control 2020, 112, 107116.
- Islam, M.A.; Karim, A.; Ethiraj, B.; Raihan, T.; Kadier, A. Antimicrobial peptides: Promising alternatives over conventional capture ligands for biosensor-based detection of pathogenic bacteria. Biotechnol. Adv. 2022, 55, 107901.
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941.
- Fong, D.; Luo, S.X.L.; Andre, R.S.; Swager, T.M. Trace ethylene sensing via wacker oxidation. ACS Cent. Sci. 2020, 6, 507–512.
- Chalupowicz, D.; Veltman, B.; Droby, S.; Eltzov, E. Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens. Actuators B Chem. 2020, 311, 127896.
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.K.; Hong, C.; Ahn, G.R.; Chung, W.J. Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sens. Actuators B Chem. 2021, 327, 128894.
- Sistani, P.; Sofimaryo, L.; Masoudi, Z.R.; Sayad, A.; Rahimzadeh, R.; Salehi, B. A penicillin biosensor by using silver nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 6201–6212.
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81.
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712.
- Igiebor, F.A.; Ikhajiagbe, B.; Asia, M. Green Nanotechnology: A modern tool for Sustainable Agriculture in Nigeria–A Review. Int. J. Hortic. Sci. Technol. 2023, 10, 269–286.
- Yadav, S.; Sawarni, N.; Dahiya, T.; Rana, J.S.; Sharma, M.; Batra, B. Nanoagriculture: Advantages and Drawbacks. In Agricultural and Environmental Nanotechnology: Novel Technologies and Their Ecological Impact; Springer Nature Singapore: Singapore, 2023; pp. 3–42.
- Boxi, S.S.; Mukherjee, K.; Paria, S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 2016, 27, 085103.
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; White, J.C. Effect of metalloid and metal oxide nanoparticles on Fusarium wilt of watermelon. Plant Dis. 2018, 102, 1394–1401.
- Sharma, R.; Garg, R.; Kumari, A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. Exp. Clin. Sci. J. 2020, 19, 1325.
- Wang, Z.; Wei, F.; Liu, S.Y.; Xu, Q.; Huang, J.Y.; Dong, X.Y.; Chen, H. Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles-modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 2020, 80, 1277–1281.
- Subbenaik, S.C. Physical and chemical nature of nanoparticles. In Plant Nanotechnology: Principles and Practices; Springer: Cham, Switzerland, 2016; pp. 15–27.
- Abu-Salah, K.M.; Zourob, M.M.; Mouffouk, F.; Alrokayan, S.A.; Alaamery, M.A.; Ansari, A.A. DNA-based nanobiosensors as an emerging platform for detection of disease. Sensors 2015, 15, 14539–14568.
- Agarwal, M.; Nagar, D.P.; Srivastava, N.; Agarwal, M.K. Chitosan Nanoparticles based Drug Delivery: An Update. Int. J. Adv. Multidiscip. Res. 2015, 2, 1–13.
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nano emulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 2020, 152, 154–170.
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. 2020, 97, 196–209.
- Sahab, A.F.; Waly, A.I.; Sabbour, M.M.; Nawar, L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. ChemTech. Res. 2015, 8, 589–598.
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55.
- Chowdappa, P.; Gowda, S.; Chethana, C.S.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812.
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnol. 2022, 20, 182.
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305.
- Wan, C.; Kahramanoğlu, İ.; Okatan, V. Application of plant natural products for the management of postharvest diseases in fruits. Folia Hortic. 2021, 33, 203–215.
- Hu, W.; Kong, H.; Guo, Y.; Zhang, Y.; Ding, Z.; Tie, W.; Guo, A. Comparative physiological and transcriptomic analyses reveal the actions of melatonin in the delay of postharvest physiological deterioration of cassava. Front. Plant Sci. 2016, 7, 736.
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integ. Plant Biol. 2021, 63, 126–145.
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2020, 13, 294.
- Li, T.; Wu, Q.; Zhu, H.; Zhou, Y.; Jiang, Y.; Gao, H.; Yun, Z. Comparative transcriptomic and metabolic analysis reveals the effect of melatonin on delaying anthracnose incidence upon postharvest banana fruit peel. BMC Plant Biol. 2019, 19, 289.
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657.
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Food Sci. 2018, 39, 226–232.
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720.
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020, 20, 231.
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Xu, L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019, 67, 2279–2288.
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. 2020, 99, 531–541.
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30.
- Palma, J.M.; Freschi, L.; Rodríguez-Ruiz, M.; González-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417.
- Tijero, V.; Munoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95.
- Arabia, A.; Munne-Bosch, S.; Muñoz, P. Melatonin triggers tissue-specific changes in anthocyanin and hormonal contents during postharvest decay of Angeleno plums. Plant Sci. 2022, 320, 111287.
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385.
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell. 2011, 23, 1795–1814.
- Di, X.; Takken, F.L.; Tintor, N. How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Front. Plant Sci. 2016, 7, 170.
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous melatonin treatment induces disease resistance against Botrytis cinerea on post-harvest grapes by activating defence responses. Foods 2022, 11, 2231.
- Li, S.; Huan, C.; Liu, Y.; Zheng, X.; Bi, Y. Melatonin induces improved protection against Botrytis cinerea in cherry tomato fruit by activating salicylic acid signaling pathway. Sci. Hortic. 2022, 304, 111299.
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to Fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish). Funct. Integr. Genom. 2015, 15, 47–62.
- Moosa, A.; Sahi, S.T.; Khan, S.A.; Malik, A.U. Salicylic acid and jasmonic acid can suppress green and blue molds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic. 2019, 31, 195–204.
- Pan, L.; Zhao, X.; Chen, M.; Fu, Y.; Xiang, M.; Chen, J. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483.
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of PpWRKY70 in the methyl jasmonate primed disease resistance against Rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466.
- Andriani, V.; Handayani, N.A. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, 87, 200–206.
- Teixeira-Costa, B.E.; Andrade, C.T. Natural polymers used in edible food packaging—History, function and application trends as a sustainable alternative to synthetic plastic. Polysaccharides 2021, 3, 32–58.
- Llanes, L.; Dubessay, P.; Pierre, G.; Delattre, C.; Michaud, P. Biosourced polysaccharide-based superabsorbents. Polysaccharides 2020, 1, 51–79.
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75.
- Kumar, N. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823.
- Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Voilley, A. Encapsulation of aroma compounds in biopolymeric emulsion based edible films to control flavour release. Carbohydr. Polym. 2010, 80, 984–988.
- Zhang, Y.; Han, J.; Liu, Z. Starch-based edible films. In Environmentally Compatible Food Packaging; Woodhead Publishing: Sawston, UK, 2008; pp. 108–136.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186.
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450.
- Firdous, N.; Moradinezhad, F.; Farooq, F.; Dorostkar, M. Advances in formulation, functionality, and application of edible coatings on fresh produce and fresh-cut products: A review. Food Chem. 2022, 407, 135186.
- Tadros, T. Surfactants. In Encyclopedia of Colloid and Interface Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1249–1250.
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745.
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527.
- Shi, Z.; Deng, J.; Wang, F.; Liu, Y.; Jiao, J.; Wang, L.; Zhang, J. Individual and combined effects of bamboo vinegar and peach gum on postharvest grey mold caused by Botrytis cinerea in blueberry. Postharvest Biol. Technol. 2019, 155, 86–93.
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020.
- Nasirifar, S.Z.; Maghsoudlou, Y.; Oliyaei, N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci. Nutr. 2018, 6, 1508–1518.
- Jahanshahi, B.; Jafari, A.; Vazifeshenas, M.R.; Gholamnejad, J. A novel edible coating for apple fruits. J. Hortic. Postharvest Res. 2018, 1, 63–72.
- Chithra, M.; Sathees, N.; Venkatesan, S.; Thirupathi, M. Effect of edible herbal coatings to extend the shelf life of banana cv. ‘Ney Poovan’ (not exposed to smoke) stored at room temperature. J. Pharmacogn. Phytochem. 2022, 11, 185–188.
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82.
- Gunny, A.A.N.; Leem, S.J.; Makhtar, M.M.Z.; Zainuddin, N.I.; Mohd Roslim, M.H.; Raja Hashim, R.H.; Rafatullah, M. The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi. Polymers 2023, 15, 2722.
- Demitri, C.; Tarantino, A.S.; Moscatello, A.; De Benedictis, V.M.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Graphene reinforced Chitosan-Cinnamaldehyde derivatives films: Antifungal activity and mechanical properties. In Proceedings of the 1st Workshop on Nanotechnology in Instrumentation and Measurement (NANOFIM), Lecce, Italy, 24–25 July 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 25–29.
- Mo, X.; Peng, X.; Liang, X.; Fang, S.; Xie, H.; Chen, J.; Meng, Y. Development of antifungal gelatin-based nanocomposite films functionalized with natamycin-loaded zein/casein nanoparticles. Food Hydrocoll. 2021, 113, 106506.
- Araújo, M.G.d.F.; Hilário, F.; Vilegas, W.; Dos Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.; Bauab, T.M. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int. J. Mol. Sci. 2012, 13, 9260–9277.
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236.
- Alvarez, M.V.; Palou, L.; Taberner, V.; Fernández-Catalán, A.; Argente-Sanchis, M.; Pitta, E.; Pérez-Gago, M.B. Natural Pectin-Based Edible Composite Coatings with Antifungal Properties to Control Green Mold and Reduce Losses of ‘Valencia’ Oranges. Foods 2022, 11, 1083.
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257.
- Salgado-Cruz, M.D.L.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421.
- Barragán-Menéndez, C.; Gálvez-López, D.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Ovando-Medina, I.; Vázquez-Ovando, A. Films of chitosan and Aloe vera for maintaining the viability and antifungal activity of Lactobacillus paracasei TEP6. Coatings 2020, 10, 259.
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2018, 34, 18–26.
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86.
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester West Sussex, UK, 2010.
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 9, 1295.
- Khan, M.R.; Chonhenchob, V.; Huang, C.; Suwanamornlert, P. Antifungal activity of propyl disulfide from neem (Azadirachta indica) in vapor and agar diffusion assays against anthracnose pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in mango fruit. Microorganisms 2021, 9, 839.
- Yin, G.; Zhang, Y.; Pennerman, K.K.; Wu, G.; Hua, S.S.T.; Yu, J.; Bennett, J.W. Characterization of blue mold Penicillium species isolated from stored fruits using multiple highly conserved loci. J. Fungi 2017, 3, 12.
- Barrera-Necha, L.L.; Bautista-Banos, S.; Flores-Moc, H.E.; Estudillo, A.R. Efficacy of Essential Oils on the Conidial Germination, Growth of Colletotrichum Gloeosporioides (Penz.) Penz. and Sacc and Control of Postharvest Diseases in Papaya (Carica Papaya L.). Plant Pathol. J. 2008, 7, 174–178.
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Schena, L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017, 66, 633–640.
- Dania, V.O.; Esiobu, M.G. Efficacy of plant-derived essential oils in post-harvest management of anthracnose disease on mango fruits. Makerere Univ. J. Agric. Environ. Sci. 2022, 11, 90–106.
- Najmi, Z.; Scalia, A.C.; De Giglio, E.; Cometa, S.; Cochis, A.; Colasanto, A.; Rimondini, L. Screening of Different Essential Oils Based on Their Physicochemical and Microbiological Properties to Preserve Red Fruits and Improve Their Shelf Life. Foods 2023, 12, 332.
- Bill, M.; Sivakumar, D.; Beukes, M.; Korsten, L. Expression of pathogenesis-related (PR) genes in avocados fumigated with thyme oil vapors and control of anthracnose. Food Chem. 2016, 194, 938–943.
- Bill, M.; Korsten, L.; Remize, F.; Glowacz, M.; Sivakumar, D. Effect of thyme oil vapors exposure on phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX) genes expression, and control of anthracnose in ‘Hass’ and ‘Ryan’ avocado fruit. Sci. Hortic. 2017, 224, 232–237.
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 2019, 9, 197.
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell wall degrading enzymes. LWT-Food Sci. Technol. 2022, 161, 113303.
